How Many Orbitals Are In The S Sublevel
listenit
Mar 19, 2025 · 5 min read
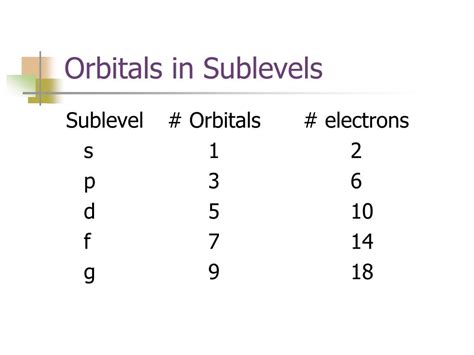
Table of Contents
How Many Orbitals Are in the s Sublevel? A Deep Dive into Atomic Structure
Understanding the structure of atoms is fundamental to comprehending chemistry. A key aspect of this understanding lies in grasping the concept of atomic orbitals and their organization within electron shells and sublevels. This article will delve into the specifics of the s sublevel, exploring how many orbitals it contains, its shape, and its significance in determining an atom's properties. We'll also touch upon related concepts like principal quantum numbers and electron configurations.
Understanding Electron Shells and Sublevels
Before diving into the s sublevel, let's establish a basic understanding of electron shells and sublevels. Electrons within an atom are not randomly distributed; they occupy specific energy levels known as shells. Each shell is designated by a principal quantum number (n), where n = 1, 2, 3, and so on, representing increasing energy levels further from the nucleus.
Within each shell, electrons are further organized into sublevels, each characterized by a specific shape and a slightly different energy level. These sublevels are designated by the letters s, p, d, and f. The number of sublevels within a shell is equal to the principal quantum number (n). For instance:
- Shell 1 (n=1): Contains only the s sublevel.
- Shell 2 (n=2): Contains the s and p sublevels.
- Shell 3 (n=3): Contains the s, p, and d sublevels.
- Shell 4 (n=4): Contains the s, p, d, and f sublevels.
The s Sublevel: Shape and Orbital Occupancy
Now, let's focus on the s sublevel. The crucial question is: how many orbitals are in the s sublevel? The answer is one. Each s sublevel can hold a maximum of two electrons, following the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers.
The s orbital itself has a spherical shape, centered on the nucleus. This spherical distribution signifies the probability of finding an electron at any given distance from the nucleus. While often depicted as a simple sphere, the reality is more nuanced. The electron's position isn't fixed; instead, the sphere represents a region of high probability density where the electron is most likely to be found. The size of the sphere increases with increasing principal quantum number (n), meaning the 2s orbital is larger than the 1s orbital.
Quantum Numbers and Orbital Designation
To completely describe an electron's location within an atom, we utilize four quantum numbers:
-
Principal Quantum Number (n): This number defines the electron shell and energy level (n = 1, 2, 3...).
-
Azimuthal Quantum Number (l): This number defines the sublevel (or subshell) and its shape. For the s sublevel, l = 0.
-
Magnetic Quantum Number (ml): This number specifies the orbital within a sublevel. For the s sublevel, since there's only one orbital, ml = 0.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum (spin) of the electron, which can be either +1/2 or -1/2.
Because the s sublevel has only one possible value for the magnetic quantum number (ml = 0), it contains only one orbital. This single orbital can accommodate a maximum of two electrons, each with opposite spins.
Electron Configuration and the s Sublevel
The electron configuration of an atom indicates how electrons are distributed among the various shells and sublevels. The s sublevel plays a crucial role in determining an atom's overall electron configuration and its chemical properties.
For example, let's consider the element lithium (Li), which has an atomic number of 3, meaning it has three electrons. Its electron configuration is 1s²2s¹. This notation indicates:
- Two electrons occupy the 1s orbital.
- One electron occupies the 2s orbital.
Similarly, beryllium (Be) with four electrons has the configuration 1s²2s². Both electrons in the 2s orbital are paired, exhibiting opposite spins.
As we progress to higher atomic numbers, more electrons fill the s orbitals of subsequent shells before filling the p, d, and f sublevels. The filling order follows the Aufbau principle, which states that electrons fill orbitals in order of increasing energy.
Significance of the s Sublevel in Chemical Bonding
The electrons in the s sublevel, particularly the valence electrons (those in the outermost shell), play a pivotal role in chemical bonding. These electrons are involved in forming chemical bonds with other atoms. The s electrons are crucial in determining an atom's reactivity and its ability to form ionic, covalent, or metallic bonds.
For example, the alkali metals (Group 1) have one electron in their outermost s sublevel, making them highly reactive. They readily lose this electron to achieve a stable electron configuration, forming positive ions. Conversely, the noble gases (Group 18) have completely filled s and p sublevels in their outermost shell, leading to their inertness and lack of reactivity.
Further Exploration: Beyond the Basics
Understanding the s sublevel is just the beginning of a deeper understanding of atomic structure and chemical behavior. Exploring further concepts, like:
- Hybridization: This describes the mixing of atomic orbitals to form new hybrid orbitals that are more suitable for bonding. s orbitals are frequently involved in hybridization.
- Molecular Orbital Theory: This theory describes the formation of molecular orbitals from atomic orbitals, further explaining the bonding in molecules.
- Spectroscopy: This experimental technique provides information about electron energy levels and transitions, confirming our understanding of orbital structure.
These advanced concepts build upon the foundational knowledge of the s sublevel and its single orbital, enriching our understanding of the complex world of atomic interactions.
Conclusion: The One and Only s Orbital
To reiterate the central theme of this article: the s sublevel contains only one orbital. This fundamental concept forms the bedrock for understanding electron configurations, chemical bonding, and the overall behavior of atoms and molecules. Understanding the properties and characteristics of the s sublevel is essential for any serious student of chemistry or physics. This single orbital, despite its apparent simplicity, plays a disproportionately significant role in shaping the universe we inhabit. By mastering this core concept, we unlock a deeper appreciation for the intricate workings of the atomic realm. The seemingly simple s orbital is anything but – it's a key to understanding the complexity of the chemical world.
Latest Posts
Latest Posts
-
How Many Drops To A Ml
Mar 19, 2025
-
The Monomers Of Proteins Are Known As
Mar 19, 2025
-
What Percentage Is 2 Out Of 7
Mar 19, 2025
-
Is 87 A Composite Or Prime Number
Mar 19, 2025
-
Solve For W Where W Is A Real Number
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Orbitals Are In The S Sublevel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
