How Many Orbitals Are In The F Sublevel
listenit
Mar 24, 2025 · 5 min read
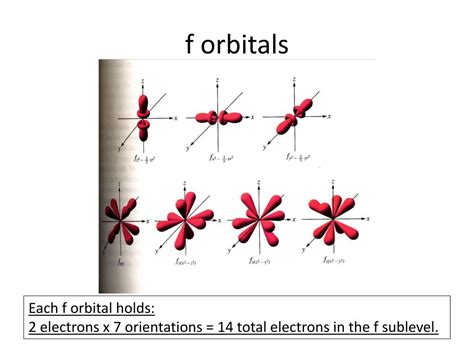
Table of Contents
How Many Orbitals Are in the f Sublevel? A Deep Dive into Atomic Structure
Understanding atomic structure is fundamental to chemistry. One key aspect of this understanding involves the arrangement of electrons within an atom, which is governed by the principles of quantum mechanics. A crucial component of this electron arrangement is the concept of sublevels, specifically, the f sublevel. This article will delve deep into the question: how many orbitals are in the f sublevel? We'll explore the underlying principles, the implications of this number, and related concepts.
Understanding Electron Configuration and Sublevels
Before we tackle the specifics of the f sublevel, let's establish a foundational understanding of electron configuration. Electrons reside in shells, subshells, and orbitals.
-
Shells: These represent the principal energy levels of electrons, denoted by the principal quantum number, n (n = 1, 2, 3...). The higher the n value, the greater the energy level and the distance from the nucleus.
-
Subshells: Within each shell, electrons are further divided into subshells, characterized by the azimuthal quantum number, l. l can take integer values from 0 to n - 1. Each value of l corresponds to a specific subshell:
- l = 0: s subshell
- l = 1: p subshell
- l = 2: d subshell
- l = 3: f subshell
-
Orbitals: Orbitals are regions of space within a subshell where there's a high probability of finding an electron. The magnetic quantum number, ml, determines the number of orbitals within a subshell. ml can take integer values from -l to +l, including 0.
The f Sublevel: Orbitals and Magnetic Quantum Number
Now, let's focus on the f sublevel. For the f subshell, l = 3. This means that the magnetic quantum number, ml, can have values ranging from -3 to +3: -3, -2, -1, 0, +1, +2, +3. Consequently, there are seven possible values for ml.
Therefore, there are seven orbitals in the f sublevel.
This is a crucial piece of information for understanding the electron configurations of heavier elements. These elements, with their many electrons, require the f orbitals to accommodate them. The filling of these orbitals plays a significant role in the chemical properties and behaviors of the lanthanides and actinides, the elements where the f orbitals are being progressively filled.
Visualizing the f Orbitals
While visualizing s, p, and even d orbitals is relatively straightforward, representing the f orbitals graphically is more challenging due to their complex shapes. However, understanding their number is still critical. Each f orbital can accommodate a maximum of two electrons, according to the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms, where ms represents the spin quantum number).
Therefore, the f sublevel, with its seven orbitals, can hold a maximum of 14 electrons (7 orbitals x 2 electrons/orbital).
Implications of Seven f Orbitals
The existence of seven f orbitals significantly impacts our understanding of:
-
The Periodic Table: The lanthanides and actinides series are characterized by the filling of the 4f and 5f orbitals, respectively. These elements exhibit similar chemical properties due to the relatively similar energies of their f electrons, a concept often referred to as the "lanthanide contraction."
-
Electronic Transitions and Spectroscopy: The energy differences between the f orbitals play a role in the electronic transitions that occur when an atom absorbs or emits light. This results in characteristic absorption and emission spectra, which can be used for identifying elements and studying their electronic structures.
-
Magnetic Properties: The unpaired electrons in the f orbitals contribute significantly to the magnetic properties of lanthanide and actinide elements. Many of these elements are paramagnetic or even ferromagnetic due to the presence of unpaired electrons in the f subshell.
-
Chemical Bonding and Reactivity: The f electrons participate in chemical bonding, although their participation is often less direct than that of s and p electrons. The extent of their involvement influences the reactivity and bonding characteristics of these elements.
Beyond the Basics: Further Exploration
While the number of orbitals within the f sublevel is definitively seven, the complexities of atomic structure continue beyond this basic understanding. For instance:
-
Relativistic Effects: In heavier elements, relativistic effects become significant, influencing the energy levels and shapes of the f orbitals. These relativistic effects can alter the chemical properties and bonding behaviors of these elements.
-
Electron Correlation: The interactions between electrons in the f orbitals also play a crucial role in determining the overall electronic structure and behavior of the atom. Accurate calculations considering electron correlation are computationally demanding.
-
Applications in Materials Science: The unique properties of lanthanides and actinides, arising from their f electron configurations, lead to their use in various materials science applications. These include high-strength magnets, catalysts, and components in high-tech devices.
Conclusion: The Significance of the Seven f Orbitals
The seemingly simple answer – seven orbitals in the f sublevel – holds profound implications for our understanding of atomic structure and the properties of matter. This knowledge is critical for chemists, physicists, and materials scientists. The complex interplay of electron-electron interactions within the f orbitals and relativistic effects creates a rich tapestry of behavior, making the study of these elements a constant source of fascination and research. The seven f orbitals are not merely a numerical fact; they are a fundamental key unlocking the properties of a substantial portion of the periodic table and their technological applications. Further research continues to unravel the intricate details of these fascinating orbitals, promising future advances in various scientific fields.
Latest Posts
Latest Posts
-
A Column Of The Periodic Table Is Called A
Mar 25, 2025
-
Derivative Of Square Root Of 3x
Mar 25, 2025
-
A Quadrilateral With Two Pairs Of Parallel Sides Is A
Mar 25, 2025
-
1 Divided By 3 4 As A Fraction
Mar 25, 2025
-
What Is The Least Common Multiple Of 14 And 6
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Orbitals Are In The F Sublevel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
