How Many Moles Are There In 17.5 Grams Of Sodium
listenit
May 12, 2025 · 4 min read
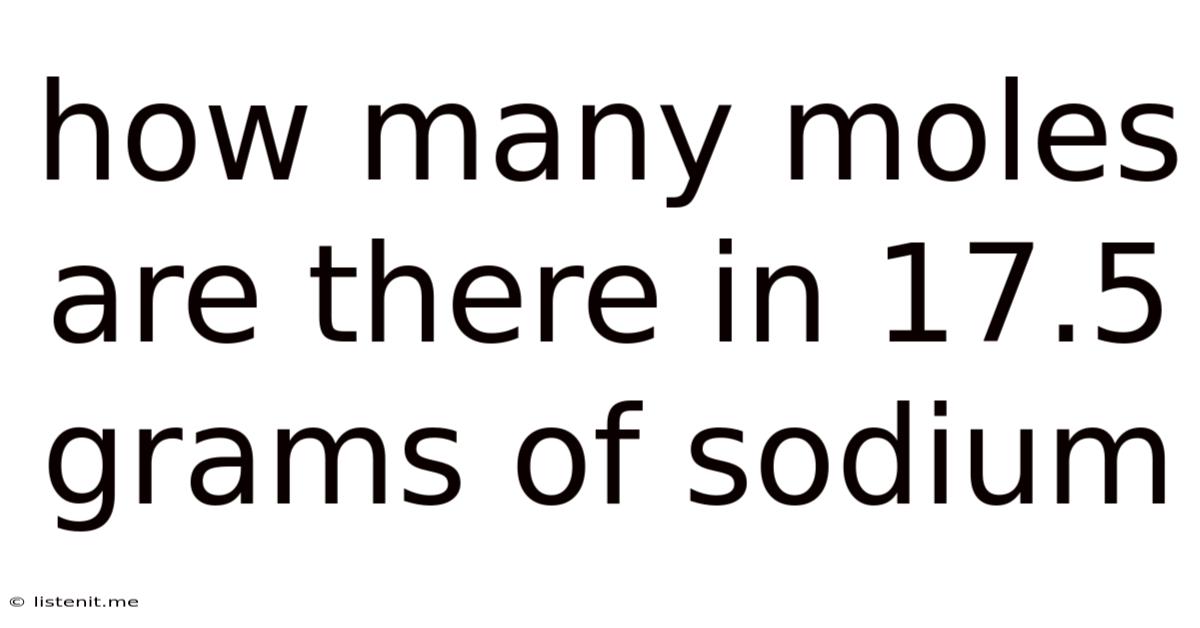
Table of Contents
How Many Moles Are There in 17.5 Grams of Sodium? A Comprehensive Guide
Understanding moles is fundamental to chemistry. This seemingly simple question – "How many moles are there in 17.5 grams of sodium?" – opens the door to a deeper understanding of stoichiometry, molar mass, and the very foundation of chemical calculations. This comprehensive guide will not only answer this question but also explore the underlying concepts, providing you with a robust understanding of molar calculations.
Understanding Moles: The Chemist's Dozen
In chemistry, the mole (mol) is a fundamental unit representing a specific number of particles, whether atoms, molecules, ions, or formula units. This number, known as Avogadro's number, is approximately 6.022 x 10<sup>23</sup>. Think of it as a chemist's "dozen"—just like a dozen eggs represents 12 eggs, a mole represents 6.022 x 10<sup>23</sup> particles.
The beauty of the mole lies in its ability to connect the macroscopic world (grams, liters) we observe with the microscopic world (atoms, molecules) that governs chemical reactions. It provides a bridge between mass and the number of particles involved.
Molar Mass: The Key to Conversion
To determine the number of moles in a given mass of a substance, we need its molar mass. The molar mass is the mass of one mole of a substance, usually expressed in grams per mole (g/mol). It's numerically equivalent to the atomic mass (for individual elements) or the sum of atomic masses (for compounds) found on the periodic table.
For sodium (Na), the atomic mass is approximately 22.99 g/mol. This means that one mole of sodium atoms has a mass of approximately 22.99 grams.
Calculating Moles: A Step-by-Step Approach
Now, let's tackle the central question: How many moles are there in 17.5 grams of sodium?
We'll use the following formula:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
-
Identify the given information: We know the mass of sodium is 17.5 grams.
-
Find the molar mass: From the periodic table, the molar mass of sodium (Na) is approximately 22.99 g/mol.
-
Apply the formula:
Moles (mol) = 17.5 g / 22.99 g/mol
Moles (mol) ≈ 0.761 mol
Therefore, there are approximately 0.761 moles of sodium in 17.5 grams of sodium.
Beyond the Calculation: Real-World Applications
Understanding mole calculations isn't just an academic exercise; it's crucial for various applications in chemistry and related fields:
-
Stoichiometry: In stoichiometric calculations, moles are essential for determining the quantities of reactants and products in chemical reactions. Balancing chemical equations and predicting yields rely heavily on mole conversions.
-
Titration: Titration, a common analytical technique, uses mole calculations to determine the concentration of unknown solutions. By knowing the moles of a standard solution reacting with an unknown solution, we can calculate the unknown's concentration.
-
Solution Preparation: Preparing solutions of specific concentrations often requires calculating the number of moles needed and then dissolving that amount in a specific volume of solvent.
-
Gas Laws: The ideal gas law (PV = nRT) uses the number of moles (n) to relate pressure (P), volume (V), temperature (T), and the ideal gas constant (R).
-
Pharmaceutical and Industrial Chemistry: Precise calculations involving moles are critical in manufacturing drugs, fertilizers, and many other products, ensuring accurate compositions and reaction yields.
Dealing with Significant Figures and Precision
In scientific calculations, paying attention to significant figures is crucial. The number of significant figures in the result should reflect the precision of the input values.
In our example, the mass of sodium (17.5 g) has three significant figures, and the molar mass of sodium (22.99 g/mol) also has four significant figures. Therefore, the result (0.761 mol) should ideally be rounded to three significant figures to maintain consistency.
Advanced Concepts: Beyond the Basics
While the basic mole calculation is straightforward, understanding its implications within more complex chemical contexts is important.
Isotopes and Average Atomic Mass
The periodic table lists the average atomic mass of an element, which accounts for the natural abundance of different isotopes. Isotopes are atoms of the same element with varying numbers of neutrons. If you're working with a specific isotope of an element, you'd use the atomic mass of that particular isotope instead of the average atomic mass.
Hydrates
Hydrates are compounds containing water molecules within their crystal structure. When calculating the molar mass of a hydrate, you must include the mass of the water molecules in the calculation.
Empirical and Molecular Formulas
Empirical formulas represent the simplest whole-number ratio of atoms in a compound, while molecular formulas represent the actual number of atoms in a molecule. Mole calculations are crucial for determining both empirical and molecular formulas from experimental data.
Conclusion: Mastering the Mole Concept
The mole is a fundamental concept in chemistry, providing a crucial link between the macroscopic and microscopic worlds. Mastering mole calculations is essential for success in chemistry and related fields. This guide has explored the basic calculation, its applications, and touched upon more advanced concepts, providing a solid foundation for further study and practical application. Remember to always pay attention to significant figures and the specific context of your calculation to ensure accuracy and precision. By understanding and applying these principles, you can confidently navigate the world of chemical calculations and unlock a deeper appreciation for the elegance and precision of chemical stoichiometry.
Latest Posts
Latest Posts
-
How Many Electrons Can 3s Hold
May 12, 2025
-
Greatest Common Factors Of 24 And 40
May 12, 2025
-
All Single Bonds Can Be Classified As
May 12, 2025
-
Fresh Water Is A Limited Resource Because
May 12, 2025
-
Miles Per Hour To Feet Per Min
May 12, 2025
Related Post
Thank you for visiting our website which covers about How Many Moles Are There In 17.5 Grams Of Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.