How Many Moles Are In 25.0 Grams Of Water
listenit
May 10, 2025 · 5 min read
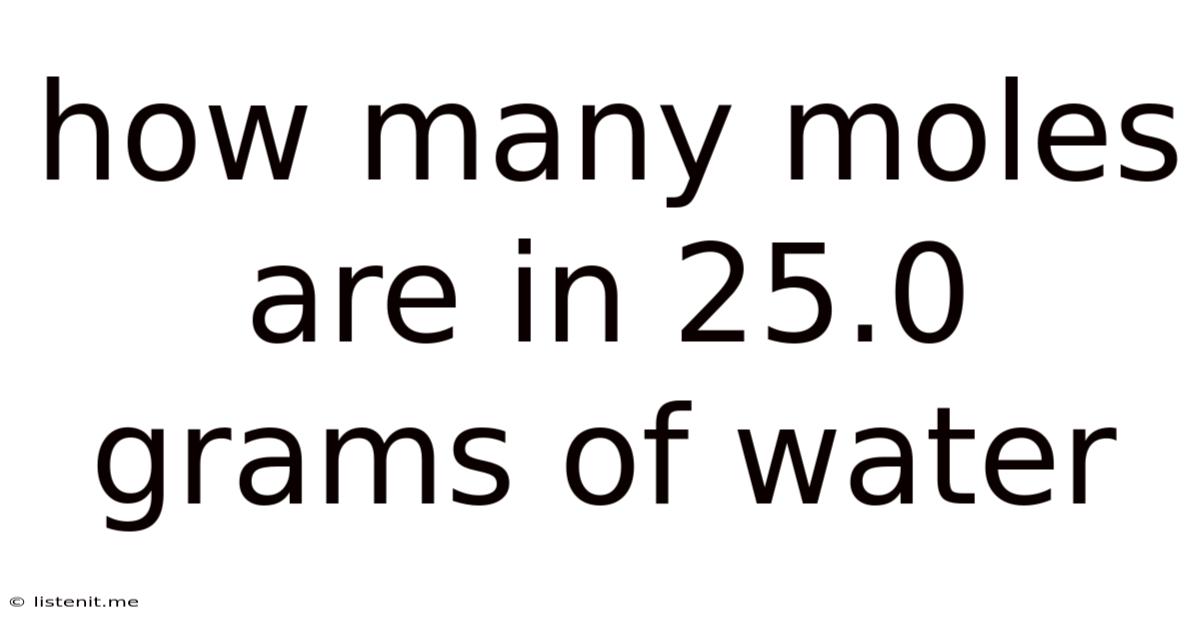
Table of Contents
How Many Moles Are in 25.0 Grams of Water? A Comprehensive Guide
Determining the number of moles in a given mass of a substance is a fundamental concept in chemistry. This article will thoroughly explore the calculation of moles in 25.0 grams of water, explaining the underlying principles, showcasing the step-by-step process, and delving into the broader implications of this calculation in various chemical contexts. We'll also touch upon related concepts and practical applications.
Understanding Moles and Molar Mass
Before diving into the calculation, let's establish a firm grasp of the key concepts involved:
What is a Mole?
A mole (mol) is the International System of Units (SI) base unit for the amount of substance. It's a crucial concept in chemistry, representing a specific number of particles, whether atoms, molecules, ions, or other entities. This number, known as Avogadro's number, is approximately 6.022 x 10<sup>23</sup>. One mole of any substance contains Avogadro's number of particles.
What is Molar Mass?
Molar mass is the mass of one mole of a substance. It's expressed in grams per mole (g/mol). The molar mass of an element is its atomic weight from the periodic table, expressed in grams. For compounds, the molar mass is the sum of the molar masses of all the atoms in the molecule.
Calculating Moles in 25.0 Grams of Water
Water (H₂O) is a simple but vital compound. To calculate the number of moles in 25.0 grams of water, we need to follow these steps:
Step 1: Determine the Molar Mass of Water
Water's molecular formula is H₂O. From the periodic table:
- The atomic mass of hydrogen (H) is approximately 1.01 g/mol.
- The atomic mass of oxygen (O) is approximately 16.00 g/mol.
Therefore, the molar mass of water is:
(2 × 1.01 g/mol) + (1 × 16.00 g/mol) = 18.02 g/mol
Step 2: Use the Mole Formula
The fundamental formula to calculate the number of moles (n) is:
n = mass (m) / molar mass (M)
Where:
- n = number of moles
- m = mass of the substance in grams
- M = molar mass of the substance in g/mol
Step 3: Plug in the Values
We know:
- m = 25.0 g
- M = 18.02 g/mol
Substituting these values into the formula:
n = 25.0 g / 18.02 g/mol
n ≈ 1.39 moles
Therefore, there are approximately 1.39 moles in 25.0 grams of water.
Significance and Applications
The ability to convert between mass and moles is crucial in numerous chemical calculations and applications:
Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Knowing the number of moles allows us to precisely determine the amounts of reactants needed or products formed in a reaction, based on the balanced chemical equation. For example, in a reaction involving water, knowing the number of moles of water present helps predict the yield of other products or reactants.
Solution Chemistry
In solution chemistry, the concentration of a solute is often expressed in terms of molarity (moles per liter). Calculating the number of moles is essential for preparing solutions of specific concentrations. For instance, to prepare a solution of a particular molarity using water as a solvent, the number of moles of the solute is required to accurately determine the mass of the solute to be dissolved.
Thermodynamics
Thermodynamic calculations, such as enthalpy changes and entropy changes, often require the number of moles as a crucial input parameter. Calculations involving heat capacities or Gibbs free energy necessitate this conversion, highlighting the importance of mole calculations in understanding energy changes in chemical processes.
Gas Laws
The ideal gas law (PV = nRT) directly relates the pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). Determining the number of moles is fundamental to predicting gas behavior under various conditions. This holds immense importance in industrial settings or studying atmospheric phenomena.
Advanced Concepts and Considerations
Isotopes and Isotopic Abundance
The molar mass of an element used in calculations is often the weighted average of the masses of its isotopes, taking into account their relative abundances. For high-precision work, the specific isotopic composition of the water sample might need to be considered. Variations in the natural abundance of deuterium (²H) and oxygen-18 (¹⁸O) isotopes, for instance, could slightly alter the molar mass and, therefore, the number of moles calculated.
Non-Ideal Behavior
The calculations described above assume ideal behavior. However, in certain conditions (e.g., high pressure, low temperature), the behavior of substances may deviate from ideality. Accurate calculations under such non-ideal conditions require more sophisticated techniques and may involve using activity coefficients or fugacity.
Experimental Error
Experimental measurements always contain some degree of uncertainty. The mass measurement of the water sample might have an associated error, leading to a slight variation in the calculated number of moles. Accurate weighing practices and appropriate significant figures are important to minimize errors.
Conclusion
Calculating the number of moles in 25.0 grams of water involves a straightforward yet fundamental calculation in chemistry. The process leverages the concept of molar mass and the mole formula, providing a cornerstone for numerous applications across various chemical disciplines. Understanding this calculation is crucial for mastering stoichiometry, solution chemistry, and many other areas. While the calculation itself is relatively simple, it's vital to keep in mind potential factors that might influence the results, such as isotopic variations and non-ideal behavior, especially when striving for high accuracy. Precise measurements and a careful understanding of the underlying concepts are crucial for reliable results. The ability to perform this calculation efficiently and accurately is a fundamental skill for any aspiring chemist or scientist.
Latest Posts
Latest Posts
-
Graph Shows A System Of Equations With Infinitely Many Solutions
May 10, 2025
-
In What Unit Is Power Measured
May 10, 2025
-
Is The Boiling Point A Physical Property
May 10, 2025
-
Is H2po4 An Acid Or Base
May 10, 2025
-
Which System Of Equations Has Two Solutions
May 10, 2025
Related Post
Thank you for visiting our website which covers about How Many Moles Are In 25.0 Grams Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.