How Many Grams Of Nh4cl Can Dissolve At 40c
listenit
Mar 12, 2025 · 5 min read
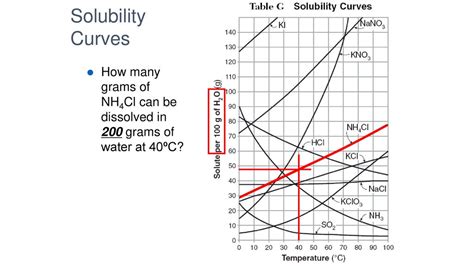
Table of Contents
How Many Grams of NH₄Cl Can Dissolve at 40°C? Exploring Solubility Curves and Factors Affecting Dissolution
Determining the exact amount of ammonium chloride (NH₄Cl) that can dissolve in water at 40°C requires understanding solubility and the factors influencing it. While a precise figure requires experimental measurement under specific conditions, we can explore the concept of solubility, analyze solubility curves, and discuss factors that affect the solubility of NH₄Cl, providing a well-rounded understanding of this chemical process.
Understanding Solubility
Solubility refers to the maximum amount of a solute (in this case, NH₄Cl) that can dissolve in a given amount of solvent (water) at a specific temperature and pressure to form a saturated solution. This is usually expressed in grams of solute per 100 grams of water (g/100g H₂O) or other units like molarity (moles/liter). When more solute is added than can dissolve, the excess remains undissolved at the bottom of the container. The solution is then described as saturated. If less solute is present, the solution is unsaturated. A supersaturated solution contains more solute than it can normally hold at a given temperature, often achieved by carefully cooling a saturated solution. However, this state is unstable, and any disturbance can cause the excess solute to precipitate out.
Solubility Curves and NH₄Cl
Solubility curves graphically represent the relationship between the solubility of a substance and temperature. These curves are typically generated through experimental data. By plotting the solubility (in g/100g H₂O) on the y-axis and temperature (°C) on the x-axis, we can visually determine the solubility of a substance at different temperatures. While a precise solubility value for NH₄Cl at 40°C can't be provided without a specific reference to an experimental solubility curve, we can discuss the general trend of NH₄Cl solubility.
Key characteristics of NH₄Cl solubility:
- Positive temperature dependence: The solubility of NH₄Cl generally increases with temperature. This means that more NH₄Cl can dissolve in hot water compared to cold water. This is a common characteristic for many ionic compounds, although not universal.
- Relatively high solubility: Ammonium chloride is a relatively soluble salt compared to some other ionic compounds. This means a significant amount can dissolve even in a relatively small volume of water.
- Non-linear relationship: The increase in solubility with temperature is not necessarily linear. The rate of increase might change as the temperature rises.
Interpreting a Solubility Curve:
To find the solubility of NH₄Cl at 40°C, locate 40°C on the x-axis of the solubility curve and follow a vertical line up until it intersects the NH₄Cl solubility curve. Then, follow a horizontal line from the intersection point to the y-axis to read the solubility in g/100g H₂O.
Absence of a Universal Curve: The exact shape and values on a solubility curve are dependent on the experimental conditions and the purity of the substances used. Different sources might provide slightly different curves.
Factors Affecting the Solubility of NH₄Cl
Several factors can affect the solubility of NH₄Cl beyond just temperature:
1. Temperature: The dominant factor.
As discussed, temperature is the primary factor influencing NH₄Cl's solubility. Higher temperatures generally lead to increased kinetic energy of water molecules, facilitating the breaking of ionic bonds in NH₄Cl and its subsequent dissolution.
2. Pressure: A minor factor for solids in liquids.
Pressure has a negligible effect on the solubility of solid solutes like NH₄Cl in liquid solvents like water. The effect becomes noticeable only at very high pressures.
3. Presence of Other Ions (Common Ion Effect):
The presence of other ions in the solution, especially those containing a common ion (NH₄⁺ or Cl⁻), can decrease the solubility of NH₄Cl. This is known as the common ion effect. The added ions shift the equilibrium towards the undissolved NH₄Cl, reducing its solubility.
4. pH:
While not a primary factor, the pH of the solution can influence the solubility of NH₄Cl to a small extent, especially if other reactions are occurring that involve the ammonium or chloride ions.
5. Impurities:
The presence of impurities in either the NH₄Cl or the water can affect solubility. Impurities might interact with the ions, affecting the dissolution process.
6. Particle Size:
Smaller particles of NH₄Cl dissolve faster than larger ones due to a larger surface area to volume ratio. However, the ultimate solubility (the maximum amount that can dissolve) remains the same regardless of particle size.
Practical Considerations and Applications
Understanding the solubility of NH₄Cl is crucial in various applications:
- Chemical synthesis: Accurate solubility data ensures the correct amount of NH₄Cl is used in chemical reactions.
- Analytical chemistry: Solubility helps in the preparation of standard solutions and understanding the behaviour of NH₄Cl in various analytical techniques.
- Agriculture: NH₄Cl is used as a fertilizer, and knowing its solubility helps in determining the appropriate concentration for application.
- Medicine: NH₄Cl has certain medicinal applications, and its solubility is essential in formulating effective dosage forms.
Conclusion: A Numerical Value is Context-Dependent
While a precise numerical value for the grams of NH₄Cl that can dissolve in 100g of water at 40°C cannot be definitively stated without referencing a specific solubility curve derived from experimental data under controlled conditions, we've explored the concept of solubility, analyzed factors affecting it, and highlighted the importance of understanding solubility curves for accurate predictions. Remember that even within experiments, slight variations are possible. Consulting a reliable chemistry handbook or scientific database will yield the most accurate experimental data for this specific solubility. The overarching understanding of solubility principles and influencing factors, however, remains crucial in numerous scientific and practical applications.
Latest Posts
Latest Posts
-
How Many Minutes Are In One Week
Mar 12, 2025
-
Where Are Fossil Fuels Not Available
Mar 12, 2025
-
1 3y 1 4 5 12
Mar 12, 2025
-
What Is 6 As A Percentage Of 20
Mar 12, 2025
-
Which Of The Following Is Insoluble In Water
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about How Many Grams Of Nh4cl Can Dissolve At 40c . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
