How Many Electrons Does The Fourth Energy Level Hold
listenit
Mar 22, 2025 · 5 min read
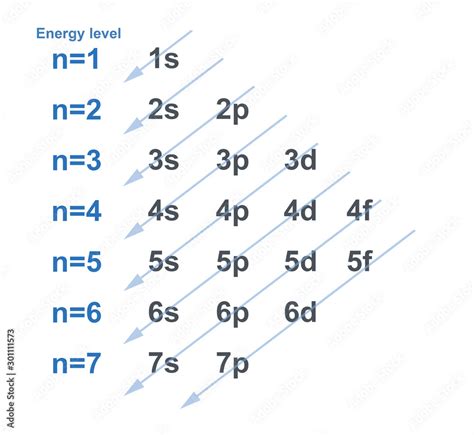
Table of Contents
How Many Electrons Does the Fourth Energy Level Hold? A Deep Dive into Electron Configuration
Understanding electron configuration is fundamental to comprehending the behavior of atoms and the properties of elements. A key aspect of this is knowing how many electrons each energy level can hold. This article delves deep into the fourth energy level, explaining not only its electron capacity but also the underlying principles governing electron arrangement within atoms.
Understanding Electron Shells and Subshells
Before we dive into the fourth energy level, let's establish the foundational concepts. Electrons, negatively charged particles, orbit the atom's nucleus in specific energy levels, often visualized as shells. These shells are not physical orbits but rather represent regions of space where the probability of finding an electron is high. Each shell is further divided into subshells, denoted by the letters s, p, d, and f. These subshells represent different regions within the shell with varying shapes and energy levels.
Key Concepts to Remember:
- Principal Quantum Number (n): This number designates the energy level or shell. It's a positive integer (1, 2, 3, 4, etc.). A higher 'n' value indicates a higher energy level and greater distance from the nucleus.
- Azimuthal Quantum Number (l): This number defines the subshell within a given shell. It ranges from 0 to n-1. 0 corresponds to the s subshell, 1 to p, 2 to d, and 3 to f.
- Magnetic Quantum Number (ml): This specifies the orbital orientation within a subshell. It ranges from -l to +l, including 0.
- Spin Quantum Number (ms): This describes the intrinsic angular momentum of an electron, either spin-up (+1/2) or spin-down (-1/2). The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers.
The Fourth Energy Level: Unveiling its Capacity
The fourth energy level (n=4) is where things start to get more complex. Unlike the first three energy levels, the fourth contains all four subshells: s, p, d, and f. Each subshell has a specific number of orbitals, and each orbital can hold a maximum of two electrons (due to the Pauli Exclusion Principle).
Subshell Breakdown for the Fourth Energy Level:
- 4s subshell (l=0): This subshell has one orbital, capable of holding a maximum of 2 electrons.
- 4p subshell (l=1): This subshell has three orbitals (px, py, pz), capable of holding a maximum of 6 electrons (2 electrons per orbital).
- 4d subshell (l=2): This subshell has five orbitals, capable of holding a maximum of 10 electrons (2 electrons per orbital).
- 4f subshell (l=3): This subshell has seven orbitals, capable of holding a maximum of 14 electrons (2 electrons per orbital).
Calculating the Total Electron Capacity:
To determine the total number of electrons the fourth energy level can hold, we simply add up the electron capacities of each subshell:
2 (4s) + 6 (4p) + 10 (4d) + 14 (4f) = 32 electrons
Therefore, the fourth energy level can accommodate a maximum of 32 electrons.
Electron Filling Order and the Aufbau Principle
The order in which electrons fill the energy levels and subshells is not simply sequential (4s before 4p, etc.). It follows the Aufbau principle, which dictates that electrons first occupy the lowest energy levels available. While generally true, there are exceptions due to the complex interplay of electron-electron repulsion and other quantum mechanical effects.
The general order of filling is often represented using the diagonal rule or Aufbau diagram. This visual aid helps to predict the electron configuration of atoms. However, it's crucial to remember that this is a guideline, and certain elements exhibit deviations from this expected pattern.
Illustrative Examples: Electron Configurations of Elements
Let's look at the electron configurations of elements that have electrons in the fourth energy level. Remember that these configurations represent the most stable arrangement of electrons for a given atom.
-
Potassium (K): [Ar] 4s¹ (Potassium has one electron in the 4s subshell after filling the 3rd energy level completely)
-
Calcium (Ca): [Ar] 4s² (Calcium has two electrons filling the 4s subshell)
-
Scandium (Sc): [Ar] 3d¹ 4s² (Here we observe a deviation in filling order. The 3d subshell starts filling before completely filling the 4th energy level because of slight differences in energy levels)
-
Krypton (Kr): [Ar] 3d¹⁰ 4s² 4p⁶ (Krypton represents a noble gas, having a full outermost shell)
These examples showcase how electrons fill the fourth energy level and the exceptions caused by the complex interactions of electrons within atoms. The electron configuration is crucial in determining the chemical properties and reactivity of an element.
Beyond the Fourth Energy Level
While we have focused on the fourth energy level, it is essential to understand that higher energy levels exist and follow similar principles. However, the complexity increases significantly. Fifth and sixth energy levels can hold even more electrons as they also contain s, p, d, and f subshells and even a g subshell theoretically in the later energy levels although not encountered in naturally occurring elements.
Conclusion: The Significance of Electron Configuration
Understanding the electron capacity of energy levels, particularly the fourth, is paramount in chemistry and physics. It forms the foundation for comprehending:
- Chemical bonding: The number of valence electrons (electrons in the outermost shell) determines an element's reactivity and how it forms bonds with other atoms.
- Periodic properties: The periodic table's organization directly reflects the filling order of electrons in energy levels and subshells.
- Spectroscopy: Electron transitions between energy levels produce unique spectral lines, used to identify elements.
- Material science: Understanding electron configuration is vital in designing and developing new materials with specific properties.
This article provides a comprehensive overview of the fourth energy level and its electron capacity. It emphasizes the fundamental principles governing electron configuration, illustrating the concepts with examples and highlighting the importance of understanding this crucial aspect of atomic structure. While the specifics of electron filling can be complex, grasping the basic principles is key to unlocking a deeper understanding of the world around us at the atomic level.
Latest Posts
Latest Posts
-
How Many Combinations In 5 Numbers
Mar 23, 2025
-
Square Root Of 85 To The Nearest Tenth
Mar 23, 2025
-
Common Factor Of 24 And 32
Mar 23, 2025
-
Which Intermolecular Force Is The Strongest
Mar 23, 2025
-
How Many Ribs Does A Pig Have
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does The Fourth Energy Level Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
