How Many Electrons Does Sr Have
listenit
Mar 20, 2025 · 5 min read
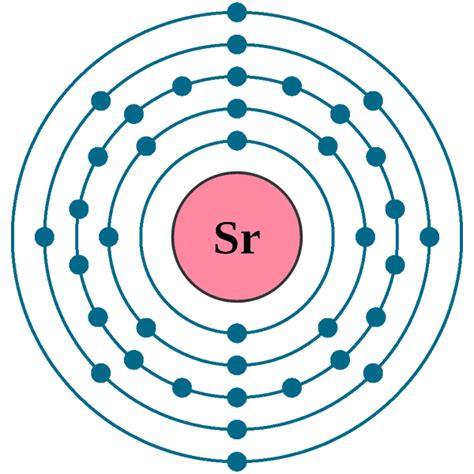
Table of Contents
How Many Electrons Does Strontium (Sr) Have? A Deep Dive into Atomic Structure
Strontium (Sr), a silvery-white alkaline earth metal, holds a fascinating place in the periodic table. Understanding its electron configuration is key to comprehending its chemical behavior and its various applications. So, how many electrons does strontium have? The answer, while seemingly simple, opens the door to a deeper exploration of atomic structure and the principles that govern the behavior of matter.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve into strontium's electron count, let's briefly review the fundamental components of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element's atomic number and its identity.
- Neutrons: Neutrally charged particles residing in the nucleus alongside protons. The number of neutrons can vary within an element, resulting in isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels. The number of electrons in a neutral atom is equal to the number of protons.
It's the electrons, specifically their arrangement and interactions, that dictate an element's chemical properties and how it bonds with other atoms.
Strontium's Atomic Number and Electron Count
Strontium's atomic number is 38. This means a neutral strontium atom contains 38 protons in its nucleus. Because a neutral atom has an equal number of protons and electrons to maintain electrical neutrality, a neutral strontium atom also possesses 38 electrons.
Isotopes and Electron Count: A Clarification
It's important to note that while the number of protons defines the element, the number of neutrons can vary. This leads to the existence of isotopes. Different isotopes of strontium will have varying numbers of neutrons, altering their atomic mass, but the number of electrons in a neutral atom of any strontium isotope remains 38. The isotopes only differ in their mass, not their chemical properties (which are primarily determined by electron configuration).
Electron Configuration: The Arrangement of Electrons
Simply stating that strontium has 38 electrons doesn't fully explain its behavior. The arrangement of these electrons in energy levels or shells is crucial. This arrangement is described by the electron configuration. For strontium, the electron configuration is:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²
Let's break down this configuration:
- Principal Energy Levels (Shells): The numbers (1, 2, 3, 4, 5) represent the principal energy levels or shells. Electrons closer to the nucleus (lower numbers) have lower energy.
- Subshells: The letters (s, p, d) represent subshells within each principal energy level. Each subshell can hold a specific number of electrons:
- s subshell: Holds a maximum of 2 electrons
- p subshell: Holds a maximum of 6 electrons
- d subshell: Holds a maximum of 10 electrons
- Superscripts: The superscripts (², ⁶, ¹⁰) indicate the number of electrons in each subshell.
This electron configuration reveals why strontium is highly reactive. The two electrons in the outermost shell (5s²) are relatively loosely bound and easily lost in chemical reactions. This is characteristic of alkaline earth metals, making strontium readily reactive.
Strontium's Chemical Behavior and its 38 Electrons
The 38 electrons of strontium, specifically the arrangement of those electrons, directly influence its chemical properties:
- Reactivity: The two valence electrons in the 5s orbital are easily lost, resulting in a +2 oxidation state. This makes strontium a highly reactive metal, readily forming ionic bonds with nonmetals.
- Oxidation States: While the +2 oxidation state is the most common, strontium can exhibit other, albeit less common, oxidation states in specific compounds.
- Bonding: Strontium primarily forms ionic compounds, donating its two valence electrons to achieve a stable electron configuration similar to noble gases (a full outermost shell).
- Reactions with Water and Acids: Strontium reacts vigorously with water and dilute acids, releasing hydrogen gas in the process. This reactivity is a direct consequence of its electron configuration and the tendency to lose its valence electrons.
Applications of Strontium and its Electron Configuration
Understanding strontium's electron configuration and its resulting properties is crucial for comprehending its various applications:
- Pyrotechnics: Strontium compounds, particularly strontium carbonate (SrCO₃), are extensively used in fireworks to produce a brilliant red color. The electron transitions within strontium atoms, when excited by heat, emit light in the red region of the visible spectrum.
- Metallurgy: Strontium is sometimes used as an alloying agent in various metals to improve their properties.
- Nuclear Medicine: Certain strontium isotopes, such as strontium-89, are used in the treatment of bone cancer.
- Phosphors: Strontium compounds are also used in the production of specific types of phosphors, materials that convert energy into light.
Further Exploration: Beyond the Basics
This article has provided a comprehensive overview of the number of electrons in strontium and the implications of its electron configuration. However, there are deeper aspects to explore, such as:
- Quantum Mechanics: A more in-depth understanding requires the principles of quantum mechanics, which explain the behavior of electrons at the atomic level.
- Spectroscopy: Studying the interaction of strontium with light (spectroscopy) provides further insights into its electron transitions and energy levels.
- Chemical Bonding Theories: Theories like Valence Bond Theory and Molecular Orbital Theory offer more sophisticated explanations of how strontium forms chemical bonds.
- Isotopic Variations: A detailed study of strontium's various isotopes and their applications in diverse fields like geology and archaeology can be undertaken.
Conclusion: The Significance of 38 Electrons
In conclusion, a neutral strontium atom possesses 38 electrons. This number, coupled with the specific arrangement of electrons detailed by its electron configuration, is the foundation of strontium's chemical behavior and its wide range of applications. From the brilliant red hues of fireworks to its role in medical treatments, understanding strontium’s 38 electrons is crucial for appreciating its significant role in various scientific and technological fields. The seemingly simple answer—38 electrons—opens a world of possibilities when explored in the context of atomic structure, chemical bonding, and the fascinating properties of this alkaline earth metal.
Latest Posts
Latest Posts
-
What Is The Si Unit Of Power
Mar 20, 2025
-
What Percent Of 18 Is 24
Mar 20, 2025
-
What Is 4 Percent Of 50
Mar 20, 2025
-
How To Find The Ph At Equivalence Point
Mar 20, 2025
-
Is Silver Tarnishing A Physical Or Chemical Change
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Sr Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
