How Many Electrons Does Scandium Have
listenit
Apr 08, 2025 · 5 min read
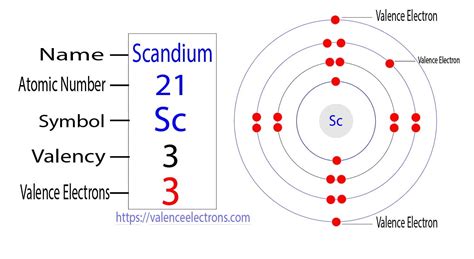
Table of Contents
How Many Electrons Does Scandium Have? A Deep Dive into Atomic Structure
Scandium, a fascinating transition metal, holds a unique position in the periodic table. Understanding its electron configuration is key to grasping its chemical properties and behavior. So, how many electrons does scandium have? The answer, simply put, is 21. But understanding why it has 21 electrons requires a deeper exploration into atomic structure, quantum numbers, and the principles governing electron arrangement.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before delving into scandium's specific electron count, let's establish a fundamental understanding of atomic structure. An atom consists of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and its identity. Scandium's atomic number is 21, meaning it has 21 protons.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within an element, leading to different isotopes.
- Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons. This is crucial for maintaining electrical neutrality.
In a neutral scandium atom, the positive charge of the 21 protons is perfectly balanced by the negative charge of 21 electrons. This balance is essential for the atom's stability.
Electron Shells and Subshells: Organizing Electrons
Electrons don't randomly orbit the nucleus. They occupy specific energy levels called shells, which are further divided into subshells. These subshells are designated by the letters s, p, d, and f, each capable of holding a specific number of electrons:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The arrangement of electrons within these shells and subshells is governed by the Aufbau principle, which dictates that electrons fill the lowest energy levels first. This principle, along with the Pauli exclusion principle (which states that no two electrons in an atom can have the same set of four quantum numbers) and Hund's rule (which dictates that electrons will individually occupy each orbital within a subshell before doubling up), determines the electron configuration.
Scandium's Electron Configuration: A Detailed Look
Now, let's apply these principles to scandium. With 21 electrons, its electron configuration is: 1s²2s²2p⁶3s²3p⁶4s²3d¹. Let's break this down:
- 1s²: The first shell (n=1) contains one s subshell, holding 2 electrons.
- 2s²: The second shell (n=2) contains one s subshell, holding 2 electrons.
- 2p⁶: The second shell also contains one p subshell, holding 6 electrons.
- 3s²: The third shell (n=3) contains one s subshell, holding 2 electrons.
- 3p⁶: The third shell also contains one p subshell, holding 6 electrons.
- 4s²: The fourth shell (n=4) contains one s subshell, holding 2 electrons. Note that the 4s subshell fills before the 3d subshell due to subtle differences in energy levels.
- 3d¹: Finally, the third shell also contains one d subshell, which in scandium holds only 1 electron.
This configuration explains scandium's properties as a transition metal. Transition metals are characterized by partially filled d orbitals, and scandium, with its single electron in the 3d subshell, perfectly fits this description.
Quantum Numbers: A More Precise Description
Electron configuration can be further refined using quantum numbers. Each electron is described by four quantum numbers:
- Principal quantum number (n): Describes the energy level or shell (n = 1, 2, 3...).
- Azimuthal quantum number (l): Describes the subshell (l = 0 for s, 1 for p, 2 for d, 3 for f).
- Magnetic quantum number (ml): Describes the orbital within the subshell (ml = -l to +l).
- Spin quantum number (ms): Describes the electron's spin (+1/2 or -1/2).
Using these quantum numbers, we can precisely specify the location and properties of each of scandium's 21 electrons.
Scandium's Chemical Behavior and Electron Configuration
Scandium's electron configuration directly influences its chemical behavior. The single electron in the 3d orbital is relatively easily lost, leading to scandium readily forming a +3 ion (Sc³⁺). This explains its reactivity and the formation of various scandium compounds. The stability of the Sc³⁺ ion, with its completely filled 3s and 3p subshells, contributes to its relatively high ionization energy after the first three electrons are removed.
Isotopes of Scandium and Electron Count
It's important to note that while a neutral scandium atom always has 21 electrons, different isotopes of scandium exist. Isotopes are variations of an element with the same number of protons but a different number of neutrons. The number of neutrons doesn't affect the electron count in a neutral atom. Therefore, regardless of the specific isotope (e.g., ⁴⁵Sc, the most common isotope), a neutral scandium atom will always possess 21 electrons.
Scandium's Role in Technology and its Relation to Electron Configuration
The unique properties of scandium, stemming directly from its electron configuration, are exploited in various technological applications. Its lightweight nature combined with high strength makes it suitable for use in alloys, particularly in aerospace applications where reducing weight is crucial. Its ability to form stable compounds makes it important in various chemical processes as well. The electron configuration determines its reactivity, leading to diverse industrial uses.
Conclusion: 21 Electrons and Beyond
In conclusion, a neutral scandium atom contains 21 electrons, a number directly dictated by its atomic number and the fundamental principles of atomic structure. Understanding this electron configuration, along with the related quantum numbers and atomic orbital concepts, is crucial for comprehending scandium's chemical properties, its reactivity, and its valuable applications across various industries. From its place on the periodic table to its unique contribution to advanced materials science, scandium's 21 electrons play a vital role in defining its identity and utility. The seemingly simple answer – 21 electrons – reveals a complex and fascinating world of atomic physics.
Latest Posts
Latest Posts
-
What Holds Two Strands Of Dna Together
Apr 08, 2025
-
What Happens To Atoms After A Chemical Change
Apr 08, 2025
-
What Decimal Is Equivalent To 1 3
Apr 08, 2025
-
Vertical Angles Are Supplements Of Each Other
Apr 08, 2025
-
What Is The Force That Holds Two Atoms Together
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Scandium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.