How Many Electrons Do Neon Have
listenit
Mar 17, 2025 · 5 min read
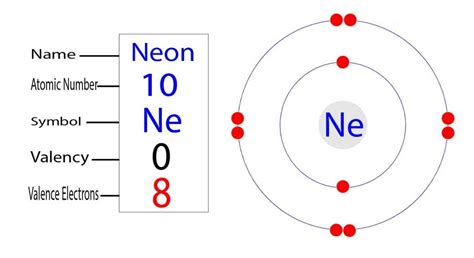
Table of Contents
How Many Electrons Does Neon Have? A Deep Dive into Atomic Structure
Neon, the vibrant gas that illuminates our signs and plays a crucial role in various technologies, holds a fascinating story within its atomic structure. Understanding the number of electrons in a neon atom is key to unlocking this story and appreciating its unique properties. This article will delve deep into the subject, exploring not only the simple answer but also the underlying principles of atomic structure and the implications of neon's electron configuration.
The Simple Answer: Neon has 10 electrons.
This is the fundamental fact we need to establish. Neon's atomic number is 10, and the atomic number represents the number of protons in an atom's nucleus. In a neutral atom, the number of protons and electrons is always equal. Therefore, a neutral neon atom possesses 10 electrons.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve deeper into neon's electron arrangement, let's review the basic building blocks of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels. The number of electrons determines the atom's chemical behavior.
The arrangement of electrons is crucial because it dictates how an atom will interact with other atoms, forming chemical bonds and molecules. This arrangement is governed by quantum mechanics, a complex field of physics that explains the behavior of matter at the atomic and subatomic levels.
Electron Shells and Subshells: Organizing Neon's Electrons
Electrons don't randomly orbit the nucleus. They occupy specific energy levels called shells, each capable of holding a limited number of electrons. These shells are further divided into subshells, designated as s, p, d, and f, each with its own unique shape and capacity.
- Shell 1 (K shell): This is the innermost shell, closest to the nucleus. It can hold a maximum of 2 electrons, filling with electrons first according to the Aufbau principle (filling the lowest energy levels first).
- Shell 2 (L shell): This shell has a capacity of 8 electrons. It's composed of one s subshell (holding 2 electrons) and one p subshell (holding 6 electrons).
- Shell 3 (M shell) and beyond: These shells can hold even more electrons, but for neon, we only need to consider the first two.
Neon's Electron Configuration: A Detailed Breakdown
Neon's 10 electrons are distributed as follows:
- Shell 1: 2 electrons (1s²)
- Shell 2: 8 electrons (2s² 2p⁶)
This is written as 1s²2s²2p⁶, which is neon's electron configuration. This configuration is incredibly stable because it represents a filled valence shell. The valence shell is the outermost shell containing electrons that participate in chemical bonding.
The Significance of a Filled Valence Shell
A filled valence shell is a key factor in determining an element's reactivity. Atoms tend to gain, lose, or share electrons to achieve a stable, filled valence shell – a concept known as the octet rule (except for the first shell, which is stable with two electrons). Neon, with its completely filled valence shell (Shell 2), is exceptionally unreactive, making it an inert gas.
Neon's Inertness and its Applications
Neon's inertness is a direct consequence of its electron configuration. It doesn't readily react with other elements because it doesn't need to gain, lose, or share electrons to achieve stability. This property underpins many of its applications:
- Neon lighting: Neon gas, when subjected to an electric current, emits a characteristic reddish-orange glow. This property is exploited in neon signs, creating vibrant and visually appealing displays. While "neon signs" are a common term, many use other gases to produce different colors.
- Helium-neon lasers: These lasers, which utilize a mixture of helium and neon, produce a highly coherent and monochromatic red light used in various applications, including barcode scanners and scientific research.
- Cryogenics: Neon's low boiling point allows its use as a cryogenic refrigerant in specialized applications requiring extremely low temperatures.
- High-voltage indicators: Neon's ability to conduct electricity at high voltages makes it useful in high-voltage indicator lamps.
Isotopes of Neon: Variations in Neutron Number
While the number of electrons determines an element's chemical properties, the number of neutrons can vary. These variations are known as isotopes. Neon has three naturally occurring stable isotopes:
- Neon-20: Contains 10 protons and 10 neutrons. This is the most abundant isotope.
- Neon-21: Contains 10 protons and 11 neutrons.
- Neon-22: Contains 10 protons and 12 neutrons.
These isotopes have the same number of electrons (10) and therefore behave chemically identically, but they differ slightly in mass.
The Quantum Mechanical Model and Neon's Electrons
The simple shell model provides a useful overview of electron arrangement, but a more accurate representation requires the quantum mechanical model. This model describes electrons as existing in atomic orbitals, which are regions of space where there's a high probability of finding an electron. These orbitals are described by quantum numbers:
- Principal quantum number (n): Corresponds to the shell number (1, 2, 3...).
- Azimuthal quantum number (l): Specifies the subshell (0 for s, 1 for p, 2 for d, 3 for f).
- Magnetic quantum number (ml): Defines the orientation of the orbital in space.
- Spin quantum number (ms): Indicates the electron's spin (+1/2 or -1/2).
Understanding neon's electron configuration within the quantum mechanical framework is essential for advanced studies in chemistry and physics.
Conclusion: The Significance of Neon's 10 Electrons
The seemingly simple answer – neon has 10 electrons – opens the door to a fascinating exploration of atomic structure, chemical behavior, and the applications of this ubiquitous element. Its stable electron configuration, with a filled valence shell, explains its inertness, a property crucial to its use in various technologies. Understanding the distribution of these 10 electrons across shells and subshells provides insight into the quantum mechanical nature of matter and the fundamental principles governing the behavior of atoms. From brightly lit signs to sophisticated scientific instruments, neon's 10 electrons play a vital role in our world.
Latest Posts
Latest Posts
-
Are The Kidneys Inferior To The Lungs
Mar 18, 2025
-
Distance From Atlanta Ga To Nashville Tennessee
Mar 18, 2025
-
What Is 80 Percent Of 12
Mar 18, 2025
-
What Is Square Root Of 45
Mar 18, 2025
-
What Is The Diameter Of A Tennis Ball
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Do Neon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
