How Many Electrons Can The P Sublevel Hold
listenit
Mar 30, 2025 · 6 min read
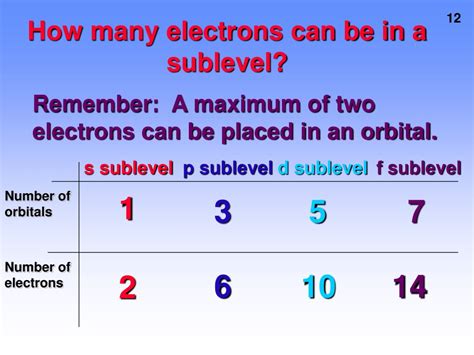
Table of Contents
How Many Electrons Can the p Sublevel Hold? A Deep Dive into Atomic Structure
Understanding the electronic structure of atoms is fundamental to chemistry and physics. A key aspect of this understanding involves knowing how many electrons can occupy each sublevel within an atom's electron shells. This article delves into the specifics of the p sublevel, explaining its capacity for electrons and the underlying principles governing this capacity.
Understanding Electron Shells and Sublevels
Before focusing on the p sublevel, let's briefly review the broader context of atomic structure. Electrons within an atom are arranged in shells, also known as energy levels. These shells are designated by principal quantum numbers (n), with n=1 representing the shell closest to the nucleus, n=2 the next, and so on. Each shell can hold a maximum number of electrons, given by the formula 2n².
However, within each shell, electrons are further organized into sublevels, also called subshells. These sublevels are distinguished by their shapes and energies and are designated by the azimuthal quantum number (l), which can have values from 0 to n-1. This leads to different types of sublevels:
- s sublevel (l=0): This sublevel is spherical in shape and can hold a maximum of two electrons.
- p sublevel (l=1): This sublevel has a dumbbell shape and can hold a maximum of six electrons. This is the focus of our article.
- d sublevel (l=2): This sublevel has a more complex shape and can hold a maximum of ten electrons.
- f sublevel (l=3): This sublevel has an even more complex shape and can hold a maximum of fourteen electrons.
The p Sublevel: Shape and Electron Capacity
The p sublevel is characterized by its dumbbell shape. Within the p sublevel, there are three p orbitals, each capable of holding a maximum of two electrons. These orbitals are oriented along the x, y, and z axes and are often denoted as p<sub>x</sub>, p<sub>y</sub>, and p<sub>z</sub>.
This three-dimensional orientation is crucial for understanding the p sublevel's capacity. Each orbital can accommodate a maximum of two electrons due to the Pauli Exclusion Principle, which states that no two electrons within an atom can have the same set of four quantum numbers. These four quantum numbers are:
- Principal quantum number (n): Specifies the energy level or shell.
- Azimuthal quantum number (l): Specifies the sublevel or subshell (s, p, d, f).
- Magnetic quantum number (m<sub>l</sub>): Specifies the orientation of the orbital within the sublevel (-l to +l, including 0). For the p sublevel, m<sub>l</sub> can be -1, 0, or +1, corresponding to p<sub>x</sub>, p<sub>y</sub>, and p<sub>z</sub> respectively.
- Spin quantum number (m<sub>s</sub>): Specifies the intrinsic angular momentum of the electron, which can be +1/2 or -1/2, often represented as "spin up" and "spin down."
Because each p orbital can hold two electrons (one with spin up and one with spin down), and there are three p orbitals, the p sublevel can accommodate a total of 2 electrons/orbital * 3 orbitals = 6 electrons.
Filling the p Sublevel: Hund's Rule and Electron Configuration
When filling the p sublevel (or any sublevel), electrons follow specific rules to achieve the lowest energy state:
- Aufbau Principle: Electrons fill orbitals starting with the lowest energy levels first.
- Hund's Rule: Within a subshell, electrons will individually occupy each orbital before doubling up in any one orbital. This minimizes electron-electron repulsion. They will also have parallel spins as much as possible.
- Pauli Exclusion Principle: As previously mentioned, no two electrons in an atom can have the same set of four quantum numbers.
Let's illustrate this with an example. Consider the element nitrogen (N), which has seven electrons. The electron configuration is 1s²2s²2p³. This means:
- The first shell (n=1) has two electrons in the 1s orbital.
- The second shell (n=2) has two electrons in the 2s orbital.
- The remaining three electrons occupy the 2p sublevel. Following Hund's rule, these three electrons occupy each of the three 2p orbitals individually, each with parallel spins, before pairing up.
Significance of the p Sublevel in Chemical Bonding
The p sublevel plays a crucial role in chemical bonding, particularly in covalent bonding. The unpaired electrons in the p orbitals are readily available to participate in the formation of covalent bonds with other atoms. This is because the sharing of electrons between atoms lowers the overall energy of the system, resulting in a more stable configuration. For example, the ability of carbon to form four bonds is directly related to its four valence electrons—two in the 2s orbital and two in the 2p orbitals.
The shape of the p orbitals also influences the geometry of molecules. For instance, the linear shape of some molecules arises from the overlap of p orbitals along a single axis. Similarly, the tetrahedral geometry of methane (CH₄) can be explained by the hybridization of s and p orbitals.
Beyond the Basics: Exceptions and Complexities
While the rules for filling electron shells and sublevels are generally well-defined, there are exceptions. Some elements show slight deviations from the predicted electron configuration, especially in transition metals and lanthanides/actinides. These deviations are often explained by factors such as the relative energy levels of different sublevels and electron-electron interactions. These exceptions highlight the complexities of atomic structure and the need for more sophisticated models to fully capture the behavior of electrons in atoms.
The p Sublevel and Spectroscopy
The p sublevel also plays a significant role in atomic spectroscopy. When atoms absorb energy, electrons can be excited to higher energy levels. When these excited electrons return to their ground state, they emit photons of specific wavelengths, which form the basis of atomic spectra. The transitions involving p orbitals contribute significantly to the observed spectral lines, making spectroscopic analysis a powerful tool for identifying elements and studying their electronic structures.
Conclusion: Mastering the p Sublevel
Understanding the capacity of the p sublevel to hold six electrons is paramount to grasping fundamental concepts in chemistry and physics. By combining the principles of quantum mechanics, the Aufbau principle, Hund's rule, and the Pauli exclusion principle, we can accurately predict the electron configurations of atoms and explain their chemical behavior. The p sublevel’s role extends beyond simple electron counting, influencing molecular shapes, bonding, and the very nature of light emitted by excited atoms. Mastering this fundamental concept unlocks a deeper comprehension of the atomic world and its intricate workings. This detailed exploration has hopefully enhanced your understanding of this essential aspect of atomic structure. Further exploration into quantum mechanics and advanced chemical theories will provide even richer insights into the fascinating world of atomic behavior.
Latest Posts
Latest Posts
-
How Long Does It Take To Drive 1500 Miles
Apr 01, 2025
-
What Is 1 6 As A Percent
Apr 01, 2025
-
11 Is What Percent Of 97
Apr 01, 2025
-
What Are The Factors For 23
Apr 01, 2025
-
How Many Membranes Surround A Chloroplast
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The P Sublevel Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
