How Many Electrons Can The Fourth Energy Level Hold
listenit
Mar 16, 2025 · 6 min read
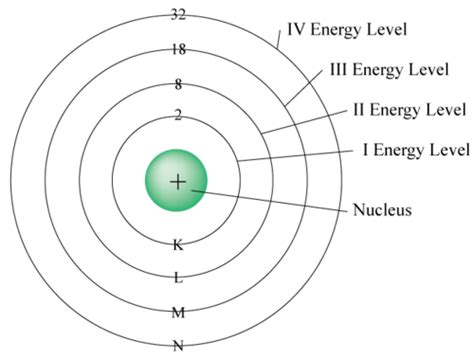
Table of Contents
How Many Electrons Can the Fourth Energy Level Hold? A Deep Dive into Atomic Structure
Understanding the electron configuration of atoms is fundamental to grasping the principles of chemistry and physics. A key aspect of this understanding involves knowing the maximum number of electrons each energy level can accommodate. This article will delve into the specifics of the fourth energy level, explaining not only the maximum electron capacity but also the underlying quantum mechanical principles that govern electron arrangement within atoms.
The Quantum Mechanical Model and Electron Shells
Before we dive into the fourth energy level, let's establish a foundational understanding of the quantum mechanical model of the atom. Unlike the simpler Bohr model, the quantum mechanical model portrays electrons not as orbiting the nucleus in defined paths, but rather existing in regions of space called orbitals. These orbitals are defined by a set of quantum numbers:
-
Principal Quantum Number (n): This number represents the energy level or shell. It's a positive integer (n = 1, 2, 3, 4...). Higher n values indicate higher energy levels and greater distance from the nucleus. This is the primary determinant of an electron's energy.
-
Azimuthal Quantum Number (l): This number describes the shape of the orbital and its angular momentum. It can have integer values from 0 to n-1. For example, if n=4, l can be 0, 1, 2, or 3. These correspond to different subshells:
- l = 0: s subshell (spherical)
- l = 1: p subshell (dumbbell-shaped)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
-
Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. It can have integer values from -l to +l, including 0. For example, if l=1 (p subshell), ml can be -1, 0, or +1, representing three p orbitals oriented along the x, y, and z axes.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often referred to as "spin." It can have only two values: +1/2 (spin up) or -1/2 (spin down). This is crucial because it dictates that each orbital can hold a maximum of two electrons, one with spin up and one with spin down – Pauli Exclusion Principle.
Calculating the Maximum Number of Electrons in the Fourth Energy Level (n=4)
Now, let's apply this knowledge to the fourth energy level (n=4). With n=4, the possible values for l are 0, 1, 2, and 3. This means the fourth energy level contains four subshells:
-
4s subshell (l=0): This subshell has one orbital (ml=0), which can hold a maximum of 2 electrons.
-
4p subshell (l=1): This subshell has three orbitals (ml = -1, 0, +1), each capable of holding 2 electrons. Therefore, the 4p subshell can hold a total of 6 electrons (3 orbitals x 2 electrons/orbital).
-
4d subshell (l=2): This subshell has five orbitals (ml = -2, -1, 0, +1, +2), capable of holding 2 electrons each. This results in a maximum of 10 electrons (5 orbitals x 2 electrons/orbital).
-
4f subshell (l=3): This subshell has seven orbitals (ml = -3, -2, -1, 0, +1, +2, +3), each holding a maximum of 2 electrons. Consequently, the 4f subshell can accommodate a total of 14 electrons (7 orbitals x 2 electrons/orbital).
To determine the total number of electrons the fourth energy level can hold, we simply sum the electron capacities of each subshell:
2 (4s) + 6 (4p) + 10 (4d) + 14 (4f) = 32 electrons
Therefore, the fourth energy level can hold a maximum of 32 electrons.
The Significance of Electron Configuration and the Fourth Energy Level
The electron configuration of an atom dictates its chemical properties and reactivity. The filling of electrons into energy levels and subshells follows specific rules, primarily the Aufbau principle (electrons fill lower energy levels first) and Hund's rule (electrons individually occupy orbitals within a subshell before pairing up).
Elements with partially filled fourth energy levels exhibit diverse chemical behavior. Transition metals, for example, have incompletely filled d orbitals in their outermost energy levels, often leading to variable oxidation states and complex coordination chemistry. Lanthanides and actinides, on the other hand, possess partially filled 4f orbitals, contributing to their unique magnetic and spectroscopic properties.
The fourth energy level plays a critical role in determining the properties of many elements crucial to life and technological advancements. Understanding its electron capacity is essential for comprehending:
-
Chemical bonding: The number of valence electrons (electrons in the outermost energy level) determines an element's bonding capacity. For elements with electrons in the fourth energy level, these valence electrons dictate the type and number of bonds they can form.
-
Spectroscopy: The electronic transitions between energy levels, including those within the fourth energy level, are responsible for the absorption and emission of light by atoms. This forms the basis of spectroscopic techniques used in chemical analysis.
-
Material science: The electronic structure of elements with electrons in the fourth energy level significantly influences the properties of materials. Understanding this allows for the design of materials with specific electrical, magnetic, or optical characteristics.
Beyond the Fourth Energy Level: Extending the Principles
The principles governing the maximum number of electrons in the fourth energy level are applicable to higher energy levels as well. The general formula for the maximum number of electrons in any energy level 'n' is 2n². However, this is a simplification, and the actual filling order is influenced by the relative energies of subshells.
While the fourth energy level holds a maximum of 32 electrons, it's crucial to understand that this number is only realized in heavier elements. Lighter elements will have fewer electrons occupying the fourth energy level, with the lower energy levels filled first.
Conclusion: The Fourth Energy Level and its Importance
In conclusion, the fourth energy level, with its capacity to hold 32 electrons, plays a pivotal role in the chemical and physical properties of numerous elements. Understanding its electron configuration, derived from the principles of quantum mechanics, is paramount for comprehending atomic behavior and the properties of matter. This knowledge forms the foundation for further exploration of chemical bonding, spectroscopy, and material science. The intricacies of electron distribution and the consequences of electron configuration underscore the power of quantum mechanics in explaining the macroscopic world around us. Through a comprehensive understanding of these principles, scientists and researchers are continually developing new materials and technologies with enhanced properties, all of which hinge on the basic structure of the atom and its electron configuration.
Latest Posts
Latest Posts
-
What Is 9 25 As A Percent
Mar 16, 2025
-
Derivative Of Ln 1 1 X
Mar 16, 2025
-
What Is The Square Root Of 130
Mar 16, 2025
-
What Is The Lcm Of 2 And 8
Mar 16, 2025
-
How To Convert Rev Sec To Rad Sec
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The Fourth Energy Level Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
