How Many Electrons Can The F Orbital Hold
listenit
Mar 29, 2025 · 4 min read
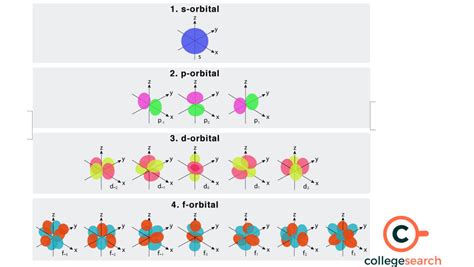
Table of Contents
How Many Electrons Can the f Orbital Hold? A Deep Dive into Atomic Structure
The question of how many electrons an f orbital can hold is fundamental to understanding atomic structure and the periodic table. While seemingly simple, the answer delves into the fascinating world of quantum mechanics and the rules governing electron behavior within atoms. This comprehensive guide will explore this question in detail, explaining the underlying principles and their implications for chemistry and physics.
Understanding Electron Orbitals
Before tackling the f orbital specifically, let's establish a foundation in atomic structure. Electrons don't simply orbit the nucleus like planets around a sun. Instead, they exist in regions of space called orbitals, defined by their energy levels and shapes. These orbitals are described by quantum numbers, which provide a mathematical framework for understanding electron behavior.
Key Quantum Numbers
Four key quantum numbers describe the properties of an electron within an atom:
-
Principal Quantum Number (n): This number dictates the energy level of the electron and the size of the orbital. It can be any positive integer (1, 2, 3...). Higher values of n correspond to higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number defines the shape of the orbital and its angular momentum. It can range from 0 to n - 1. Different values of l correspond to different subshells:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can take integer values from -l to +l, including 0. For example, a p orbital (l = 1) has three possible orientations (m<sub>l</sub> = -1, 0, +1).
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often visualized as "spin up" (+1/2) or "spin down" (-1/2). This is an intrinsic property of the electron itself, not related to its orbital motion.
The f Orbital: Shape and Complexity
The f orbital, characterized by l = 3, is the most complex orbital type. Its shape is difficult to visualize accurately, with multiple lobes and nodal planes (regions of zero electron density). Unlike the simpler s, p, and d orbitals, the f orbitals have intricate three-dimensional structures. There are seven possible f orbitals, each with a unique orientation in space, corresponding to the seven possible values of m<sub>l</sub> (-3, -2, -1, 0, 1, 2, 3).
The Pauli Exclusion Principle and Electron Capacity
The Pauli Exclusion Principle is crucial in determining how many electrons an orbital can hold. This principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that within a single orbital, a maximum of two electrons can exist, provided they have opposite spins (one with ms = +1/2 and the other with ms = -1/2).
How Many Electrons Can an f Subshell Hold?
Since there are seven f orbitals, and each orbital can hold a maximum of two electrons (due to the Pauli Exclusion Principle), the f subshell can hold a total of 14 electrons. This is why the f-block elements in the periodic table have 14 elements per row. These elements are also known as the lanthanides and actinides.
Implications for the Periodic Table and Chemical Properties
The capacity of the f orbital to hold 14 electrons directly impacts the structure and properties of the periodic table. The filling of the f orbitals leads to the lanthanide and actinide series, with elements exhibiting similar chemical properties due to the shielding effect of the filled inner f orbitals. The subtle differences in chemical behavior across these series are attributed to the intricate interactions between the f electrons and the outer electrons, which participate in chemical bonding.
Advanced Concepts and Considerations
While the basic answer is 14 electrons, a deeper understanding requires considering:
-
Electron Configuration and Hund's Rule: Electrons fill orbitals according to specific rules. Hund's Rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This influences the magnetic properties of atoms and ions.
-
Relativistic Effects: In heavier atoms, relativistic effects become significant, altering the energies of the electrons and influencing the properties of the elements. These effects are particularly important for the f-block elements.
-
Orbital Hybridization: In chemical bonding, atomic orbitals can combine to form hybrid orbitals with different shapes and energies. This process can significantly influence molecular geometry and reactivity.
Conclusion: 14 Electrons and Beyond
The f orbital, with its complex structure and ability to hold 14 electrons, plays a vital role in shaping the periodic table and the properties of the elements. Understanding its electron capacity requires a solid grasp of quantum mechanics and the principles governing electron behavior within atoms. The seemingly simple question of "how many electrons can the f orbital hold?" opens a window into the fascinating world of atomic structure and its profound impact on chemistry and physics. The implications extend far beyond the basic answer, enriching our understanding of the chemical world around us. Further exploration of relativistic effects, electron configuration, and orbital hybridization provides a more comprehensive and nuanced understanding of this fundamental concept.
Latest Posts
Latest Posts
-
Gcf Of 42 126 And 210
Apr 01, 2025
-
What Are The First 5 Multiples Of 7
Apr 01, 2025
-
What Is 8 10 As A Decimal
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The F Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
