How Many Electrons Are In Potassium
listenit
Mar 13, 2025 · 6 min read
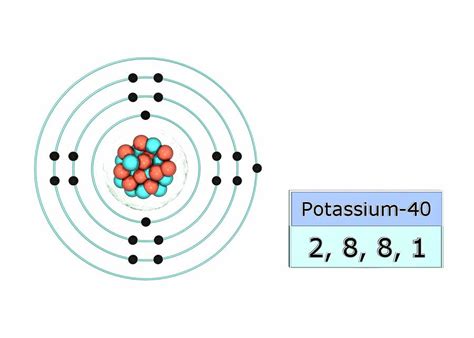
Table of Contents
How Many Electrons Are in Potassium? A Deep Dive into Atomic Structure
Potassium, a vital element for life, plays a crucial role in various biological processes. Understanding its atomic structure, particularly the number of electrons it possesses, is fundamental to comprehending its chemical behavior and biological significance. This article will delve into the intricacies of potassium's electron configuration, exploring its position on the periodic table, its role in chemical bonding, and its importance in biological systems. We'll also touch upon related concepts, like isotopes and ionization, to provide a comprehensive understanding.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we determine the number of electrons in potassium, let's briefly review the fundamental components of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; all potassium atoms have 19 protons.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes (discussed later).
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
Potassium's Position on the Periodic Table
Potassium (K), with atomic number 19, is located in the fourth period and Group 1 (alkali metals) of the periodic table. Its position provides crucial information about its electronic structure. The atomic number, 19, directly tells us the number of protons and, in a neutral atom, the number of electrons.
Therefore, a neutral potassium atom has 19 electrons.
Electron Configuration: Distributing Electrons in Shells
Electrons don't randomly orbit the nucleus; they occupy specific energy levels or shells. These shells have a maximum capacity for electrons, determined by the formula 2n², where 'n' is the shell number (1, 2, 3, etc.). The electron configuration describes how electrons are distributed among these shells.
Potassium's electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹. Let's break this down:
- 1s²: Two electrons in the first shell (n=1). The 's' subshell can hold a maximum of two electrons.
- 2s²: Two electrons in the second shell (n=2), 's' subshell.
- 2p⁶: Six electrons in the second shell (n=2), 'p' subshell. The 'p' subshell can hold a maximum of six electrons.
- 3s²: Two electrons in the third shell (n=3), 's' subshell.
- 3p⁶: Six electrons in the third shell (n=3), 'p' subshell.
- 4s¹: One electron in the fourth shell (n=4), 's' subshell.
This configuration explains potassium's chemical reactivity. The single electron in the outermost shell (4s¹) is easily lost, resulting in a +1 ion (K⁺). This tendency to lose an electron makes potassium highly reactive and readily forms ionic bonds.
Potassium's Reactivity and Chemical Bonding
The single valence electron in potassium's outermost shell is responsible for its high reactivity. Potassium readily loses this electron to achieve a stable octet (eight electrons) in its outermost shell, a configuration that mimics the noble gases and represents a state of low energy. This electron loss forms a K⁺ cation (positive ion).
Potassium's reactivity leads to the formation of ionic compounds with non-metals, like chlorine (Cl). Potassium readily donates its valence electron to chlorine, forming potassium chloride (KCl), a common salt. The electrostatic attraction between the positively charged potassium ion (K⁺) and the negatively charged chloride ion (Cl⁻) forms the ionic bond.
Isotopes of Potassium: Variations in Neutron Number
While the number of protons defines an element, the number of neutrons can vary. Atoms of the same element with different neutron numbers are called isotopes. Potassium has three naturally occurring isotopes: ³⁹K, ⁴⁰K, and ⁴¹K. The superscript represents the mass number (protons + neutrons).
Even though these isotopes have different numbers of neutrons, they all have 19 protons and, in their neutral state, 19 electrons. The differences in neutron numbers affect the atomic mass and radioactive properties of the isotopes. ⁴⁰K, for instance, is a naturally occurring radioactive isotope.
Biological Significance of Potassium
Potassium is essential for life, playing a vital role in numerous biological processes:
- Maintaining fluid balance: Potassium helps regulate the balance of fluids inside and outside cells.
- Nerve impulse transmission: Potassium ions are crucial for the transmission of nerve impulses. The movement of potassium ions across cell membranes is essential for generating action potentials.
- Muscle contraction: Similar to nerve impulse transmission, potassium ions are vital for muscle contraction. Changes in potassium concentration across muscle cell membranes trigger muscle contractions.
- Enzyme activity: Potassium acts as a cofactor for several enzymes, meaning it is essential for their proper functioning.
Imbalances in potassium levels can lead to serious health problems, highlighting its critical role in maintaining overall health.
Ionization of Potassium: Losing Electrons
Ionization is the process of removing or adding electrons to an atom, resulting in the formation of an ion. Potassium's low ionization energy makes it relatively easy to remove its single valence electron, forming the K⁺ ion. This cation is essential for various biological functions as mentioned earlier.
The first ionization energy of potassium is relatively low, indicating that removing the first electron requires less energy compared to other elements. Subsequent ionization energies are significantly higher because removing electrons from inner shells requires more energy to overcome the stronger electrostatic attraction to the nucleus.
Potassium in Everyday Life
Potassium is ubiquitous in our daily lives, found in various sources including:
- Bananas: A well-known source of potassium.
- Potatoes: Another excellent source of this essential mineral.
- Tomatoes: Contain significant amounts of potassium.
- Leafy green vegetables: Spinach and kale are good examples.
- Dairy products: Milk and yogurt contribute to potassium intake.
- Many processed foods: Check food labels for potassium content.
Conclusion: The Importance of Understanding Potassium's Electron Count
The seemingly simple question, "How many electrons are in potassium?" leads us to a deeper exploration of atomic structure, chemical bonding, and biological significance. Understanding that a neutral potassium atom has 19 electrons is crucial for comprehending its reactivity, its role in forming ionic compounds, and its vital functions in biological systems. This knowledge underscores the importance of studying the fundamental properties of elements to understand their impact on our world, from chemical reactions to the processes sustaining life itself. The consistent number of 19 electrons in neutral potassium atoms, regardless of isotopic variations, emphasizes the fundamental nature of atomic number and its role in defining an element's properties and behavior. This understanding forms a foundation for further studies in chemistry and biology. The presence of potassium, and its 19 electrons, in various biological processes highlights its crucial role in maintaining life and health. This knowledge has far-reaching implications in various fields, including medicine and agriculture. By understanding potassium's electron count and its subsequent chemical properties, we unlock a deeper understanding of its importance in the natural world.
Latest Posts
Latest Posts
-
Whats The Thinnest Layer Of The Earth
Mar 14, 2025
-
How Do Spectrographs Help Astronomers Classify Stars
Mar 14, 2025
-
Lcm Of 4 And 5 And 3
Mar 14, 2025
-
A Student Wearing Frictionless In Line Skates
Mar 14, 2025
-
Are The Moon And The Sun The Same Thing
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
