How Many Atoms Are In Sodium
listenit
Mar 20, 2025 · 5 min read
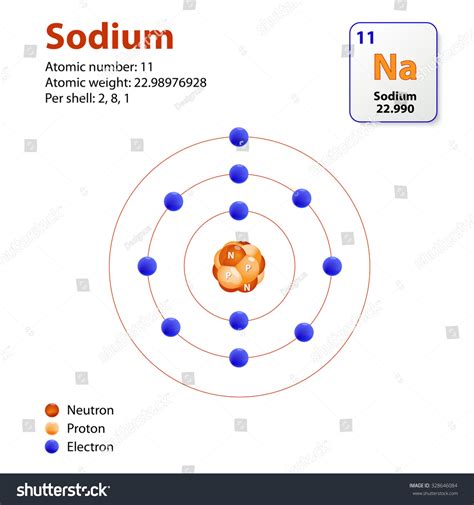
Table of Contents
- How Many Atoms Are In Sodium
- Table of Contents
- How Many Atoms Are in Sodium? A Deep Dive into Avogadro's Number and Atomic Mass
- Understanding Avogadro's Number
- The Mole Concept
- Atomic Mass and Molar Mass
- Atomic Mass
- Molar Mass
- Calculating the Number of Atoms in Sodium
- Factors Affecting Atom Count: Isotopes and Purity
- Isotopic Abundance Variations
- Impurities
- Beyond Sodium: Applying the Principles to Other Elements
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
How Many Atoms Are in Sodium? A Deep Dive into Avogadro's Number and Atomic Mass
Determining the number of atoms in a given amount of sodium requires understanding some fundamental concepts in chemistry, namely Avogadro's number and atomic mass. This article will explore these concepts, explain how to calculate the number of atoms in sodium, and delve into related topics, such as isotopes and molar mass.
Understanding Avogadro's Number
Avogadro's number, approximately 6.022 x 10<sup>23</sup>, represents the number of constituent particles (atoms, molecules, ions, etc.) in one mole of a substance. It's a cornerstone of chemistry, providing a crucial link between the macroscopic world (grams, liters) and the microscopic world (atoms, molecules). Think of it as a conversion factor, allowing us to move seamlessly between the number of particles and the mass of a substance.
The Mole Concept
The mole is the SI unit for amount of substance. One mole of any substance contains Avogadro's number of particles. This means one mole of carbon atoms contains 6.022 x 10<sup>23</sup> carbon atoms, one mole of water molecules contains 6.022 x 10<sup>23</sup> water molecules, and so on. This consistency is what makes Avogadro's number so powerful.
Atomic Mass and Molar Mass
Before we can calculate the number of atoms in a sample of sodium, we need to understand atomic mass and molar mass.
Atomic Mass
Atomic mass, also known as atomic weight, is the average mass of an atom of an element, taking into account the relative abundance of its isotopes. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This difference in neutron number leads to slight variations in mass. For example, sodium predominantly exists as <sup>23</sup>Na (sodium-23), with a small percentage of <sup>22</sup>Na (sodium-22). The atomic mass of sodium listed on the periodic table, approximately 22.99 u (unified atomic mass units), represents the weighted average of these isotopes.
Molar Mass
Molar mass is the mass of one mole of a substance. It's numerically equivalent to the atomic mass (for elements) or molecular mass (for compounds) but expressed in grams per mole (g/mol) instead of atomic mass units (u). Therefore, the molar mass of sodium is approximately 22.99 g/mol.
Calculating the Number of Atoms in Sodium
Let's now tackle the central question: how many atoms are in a given amount of sodium? The calculation involves a straightforward three-step process:
-
Determine the mass of sodium: Let's assume we have 10 grams of sodium.
-
Convert mass to moles: Using the molar mass of sodium (22.99 g/mol), we can convert the mass to moles:
- Moles of sodium = (Mass of sodium) / (Molar mass of sodium)
- Moles of sodium = 10 g / 22.99 g/mol ≈ 0.435 moles
-
Convert moles to atoms: Finally, using Avogadro's number, we can convert the number of moles to the number of atoms:
- Number of atoms = (Moles of sodium) x (Avogadro's number)
- Number of atoms ≈ 0.435 moles x 6.022 x 10<sup>23</sup> atoms/mol ≈ 2.62 x 10<sup>23</sup> atoms
Therefore, there are approximately 2.62 x 10<sup>23</sup> atoms in 10 grams of sodium.
Factors Affecting Atom Count: Isotopes and Purity
The calculation above assumes pure sodium with the standard isotopic distribution. However, several factors can influence the actual number of atoms:
Isotopic Abundance Variations
The atomic mass of sodium (22.99 u) is an average. Slight variations in the isotopic abundance of <sup>23</sup>Na and <sup>22</sup>Na in different samples of sodium could lead to minor differences in the number of atoms calculated for a given mass. These variations are usually small and often negligible for most practical purposes.
Impurities
If the sodium sample contains impurities (other elements or compounds), the mass of sodium will be less than the total mass of the sample. This would result in a lower number of sodium atoms calculated using the method described above. The presence of impurities highlights the importance of using high-purity samples for accurate atomic calculations.
Beyond Sodium: Applying the Principles to Other Elements
The method described above for calculating the number of atoms in sodium is applicable to any element. You simply need to substitute the molar mass of the element of interest and the mass of the sample. For instance:
- Gold (Au): Gold has an atomic mass of approximately 197 g/mol. If you have 5 grams of pure gold, you can calculate the number of gold atoms using the same three-step process.
- Carbon (C): Carbon has an atomic mass of approximately 12 g/mol. Similarly, you can determine the number of carbon atoms in a given mass using the same methodology.
Conclusion
Determining the number of atoms in a sample of sodium, or any element, relies on understanding the fundamental concepts of Avogadro's number, atomic mass, and molar mass. While the calculation is relatively straightforward, it's crucial to remember the potential influence of isotopic variations and impurities on the final result. The principles discussed here are fundamental to many areas of chemistry and provide a powerful tool for understanding the relationship between macroscopic quantities and the microscopic world of atoms and molecules. This knowledge is crucial for various applications ranging from materials science and nanotechnology to medicine and environmental studies. By grasping these concepts, we can move confidently between the vastness of the macroscopic world and the intricate detail of the atomic realm.
Latest Posts
Latest Posts
-
How Many Punts In A Quart
Mar 22, 2025
-
What Is The Shape Of A Planetary Orbit
Mar 22, 2025
-
How Much Is A Half Mile
Mar 22, 2025
-
What Is 9 Oz In Cups
Mar 22, 2025
-
Greatest Common Factor Of 36 And 60
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
