How Do You Make A Supersaturated Solution
listenit
Mar 29, 2025 · 6 min read
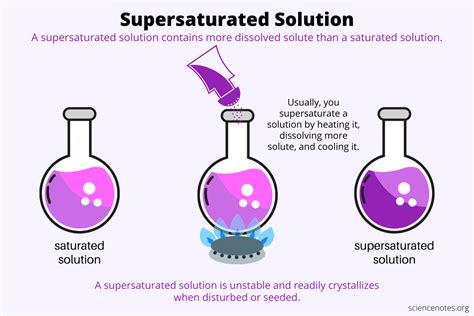
Table of Contents
How to Make a Supersaturated Solution: A Comprehensive Guide
Supersaturated solutions, fascinating examples of chemical equilibrium, represent a state where a solution holds more solute than it theoretically should at a given temperature. Understanding how to create these metastable solutions opens doors to various scientific experiments and applications. This comprehensive guide delves into the intricacies of supersaturation, explaining the process, the factors influencing it, and safety precautions to ensure successful and safe experimentation.
Understanding Supersaturation: A Deeper Dive
Before embarking on the creation of a supersaturated solution, it's crucial to grasp the underlying principles. A saturated solution is one where the solvent has dissolved the maximum amount of solute possible at a specific temperature and pressure. Any additional solute added will simply settle at the bottom, remaining undissolved.
A supersaturated solution, however, contains more solute than a saturated solution at the same temperature and pressure. This seemingly paradoxical state is inherently unstable; the solution is in a metastable equilibrium. A tiny disturbance, such as adding a seed crystal, scratching the container, or even a change in temperature, can trigger the excess solute to rapidly crystallize, resulting in a spectacular display of precipitation.
This instability stems from the fact that the dissolved solute exists in a higher energy state than its crystalline form. The crystallization process releases this excess energy, making it thermodynamically favorable.
Factors Affecting Supersaturation
Several key factors influence the formation and stability of a supersaturated solution. Understanding these factors is vital for successfully preparing one:
1. Temperature: The Key Player
Temperature plays a crucial role. Most solids exhibit increased solubility with increasing temperature. This principle is exploited in creating supersaturated solutions. The process typically involves:
- Heating: Dissolving a large amount of solute in a solvent at a high temperature. The higher temperature allows the solvent to accommodate far more solute than it would at room temperature.
- Cooling: Slowly and carefully cooling the solution to room temperature or below. The key is to avoid any disturbance during cooling, preventing premature crystallization. Slow cooling maximizes the chances of the excess solute remaining dissolved in the metastable state.
2. Solvent Selection: Choosing the Right Medium
The choice of solvent significantly impacts the success of supersaturation. Solvents with high polarity and a strong affinity for the solute generally favor higher solubility.
3. Solute Properties: The Nature of the Substance
The inherent properties of the solute, such as its crystal structure and tendency to form hydrates, influence its solubility and its propensity to supersaturate. Some solutes readily form supersaturated solutions, while others are more resistant.
4. Impurities: The Unwanted Guests
The presence of impurities can act as nucleation sites, initiating premature crystallization. Therefore, using clean glassware and high-purity chemicals is essential for maximizing the chances of successful supersaturation.
5. Rate of Cooling: The Gentle Approach
Rapid cooling increases the likelihood of crystallization. Slow, controlled cooling significantly improves the chances of maintaining the supersaturated state. This allows the solute molecules to remain in solution even when their solubility is exceeded.
Step-by-Step Guide to Creating a Supersaturated Solution
Let's outline a general procedure for making a supersaturated solution. Remember, this is a general guideline; specific quantities and temperatures will vary depending on the chosen solute and solvent. Always consult relevant safety data sheets (SDS) before handling chemicals.
Materials:
- Beaker or flask
- Hot plate or Bunsen burner (with appropriate safety precautions)
- Stirring rod or magnetic stirrer
- Thermometer
- Solute (e.g., sodium acetate, sugar)
- Solvent (e.g., water)
- Filter paper (optional, for removing impurities)
- Seed crystal (optional, for controlled crystallization)
- Safety goggles and gloves
Procedure:
-
Heating the Solvent: Carefully heat the solvent in the beaker using a hot plate or Bunsen burner. Monitor the temperature using a thermometer.
-
Adding the Solute: Gradually add the solute to the heated solvent, stirring continuously to ensure complete dissolution. Add more solute than would normally dissolve at room temperature. The solution may appear slightly cloudy initially, which is often caused by the presence of air or impurities. You may consider gently heating the solution for a longer time and filtering it to help clarify the solution.
-
Monitoring Saturation: Continue adding solute until no more dissolves and a small amount settles at the bottom of the beaker. At this stage, the solution is saturated. Adding a little more solute will help ensure you have exceeded the saturation limit.
-
Careful Cooling: Carefully remove the beaker from the heat source. Allow the solution to cool slowly to room temperature, ideally without stirring. Cover the beaker to minimize dust and air contamination. This slow cooling prevents the formation of nuclei and promotes the formation of a supersaturated solution.
-
Observing Supersaturation: Once the solution reaches room temperature, you have a supersaturated solution. It will appear clear, containing more dissolved solute than it should at that temperature. This is a metastable state that is likely to change rapidly once crystallization is initiated.
Initiating Crystallization: The Dramatic Reveal
The supersaturated solution is in a metastable equilibrium. A small trigger can initiate rapid crystallization. Several methods can be used:
-
Adding a Seed Crystal: Introducing a tiny crystal of the solute acts as a nucleation site, causing rapid crystallization of the excess solute.
-
Scratching the Container: Scratching the inside of the container creates imperfections that can serve as nucleation sites.
-
Adding a different solute: The presence of foreign ions can disrupt the equilibrium and trigger the precipitation of the dissolved solute.
-
Temperature Change: A sudden change in temperature (either increase or decrease) can destabilize the solution and trigger crystallization.
The resulting crystallization is often dramatic, with rapid formation of crystals.
Applications of Supersaturated Solutions
Supersaturated solutions find applications in various fields:
-
Chemical Synthesis: Supersaturated solutions are used to control the size and morphology of crystals in chemical synthesis and material science.
-
Crystal Growth: The controlled crystallization of supersaturated solutions is crucial in growing high-quality single crystals for various applications.
-
Medicine: Supersaturated solutions are used in some pharmaceutical formulations to improve drug solubility and bioavailability.
-
Food Science: The principles of supersaturation are applied in the production of candies and other food products.
-
Other Applications: Other applications range from creating artistic displays to scientific experiments demonstrating principles of equilibrium and kinetics.
Safety Precautions: Handling with Care
Working with supersaturated solutions requires careful attention to safety:
-
Eye Protection: Always wear appropriate safety goggles to protect your eyes from potential splashes or spills.
-
Gloves: Use chemical-resistant gloves to protect your hands from the chemicals used.
-
Proper Ventilation: Work in a well-ventilated area to minimize exposure to any potentially harmful vapors.
-
Heating Precautions: Exercise caution when using a hot plate or Bunsen burner to avoid burns.
-
Disposal: Dispose of chemicals according to local regulations and safety guidelines.
Conclusion: A Fascinating Chemical Phenomenon
Creating a supersaturated solution is a rewarding scientific exercise, showcasing the intriguing nature of chemical equilibrium and the metastable state. By understanding the underlying principles and following the safety guidelines outlined above, you can successfully prepare and observe the fascinating phenomenon of supersaturation. Remember that experimentation requires careful planning, meticulous execution, and a steadfast commitment to safety. This guide provides a robust foundation for exploring the world of supersaturated solutions and their diverse applications. Remember to always prioritize safety and consult relevant safety data sheets before handling any chemicals.
Latest Posts
Latest Posts
-
Gcf Of 42 126 And 210
Apr 01, 2025
-
What Are The First 5 Multiples Of 7
Apr 01, 2025
-
What Is 8 10 As A Decimal
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Do You Make A Supersaturated Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
