How Do You Know If A Reaction Is Spontaneous
listenit
Apr 01, 2025 · 5 min read
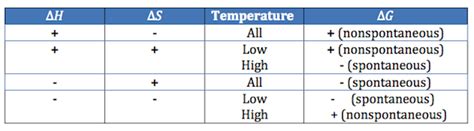
Table of Contents
How Do You Know if a Reaction is Spontaneous?
Spontaneity, in the context of chemical reactions, doesn't mean a reaction happens quickly or explosively. Instead, it refers to whether a reaction will proceed without external intervention once initiated. A spontaneous reaction will proceed on its own towards equilibrium, while a non-spontaneous reaction requires continuous input of energy to occur. Understanding spontaneity is crucial in chemistry, thermodynamics, and many other fields. This comprehensive guide will explore the various ways we determine if a reaction is spontaneous, focusing on the key concepts and their practical applications.
The Role of Entropy and Enthalpy
The spontaneity of a reaction is governed primarily by two thermodynamic properties: enthalpy (ΔH) and entropy (ΔS).
Enthalpy (ΔH): The Heat Factor
Enthalpy represents the heat content of a system at constant pressure. A negative ΔH (ΔH < 0) indicates an exothermic reaction, where heat is released to the surroundings. These reactions are often favored because they release energy, making them more likely to occur spontaneously. Think of burning wood – the heat released makes the reaction spontaneous.
A positive ΔH (ΔH > 0) indicates an endothermic reaction, where heat is absorbed from the surroundings. These reactions require an input of energy to proceed, and are generally less likely to be spontaneous. Melting ice, for example, requires heat input, making it a non-spontaneous process at temperatures below 0°C.
Entropy (ΔS): The Disorder Factor
Entropy measures the randomness or disorder of a system. A positive ΔS (ΔS > 0) indicates an increase in disorder, meaning the products are more disordered than the reactants. For example, the expansion of a gas into a vacuum leads to increased disorder, resulting in a positive ΔS.
A negative ΔS (ΔS < 0) indicates a decrease in disorder, meaning the products are more ordered than the reactants. The formation of a crystalline solid from its constituent atoms is an example of a process with negative ΔS.
Gibbs Free Energy: The Decisive Factor
While enthalpy and entropy provide valuable insights, they don't individually determine spontaneity. Instead, the Gibbs Free Energy (ΔG) combines both factors to provide a definitive answer. It's defined by the equation:
ΔG = ΔH - TΔS
where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy
- T is the absolute temperature (in Kelvin)
- ΔS is the change in entropy
The sign of ΔG dictates the spontaneity of a reaction at constant temperature and pressure:
- ΔG < 0: The reaction is spontaneous (favored) under the given conditions.
- ΔG > 0: The reaction is non-spontaneous (unfavored) under the given conditions. Energy input is required.
- ΔG = 0: The reaction is at equilibrium; the forward and reverse reaction rates are equal.
Temperature's Influence on Spontaneity
Notice the temperature (T) in the Gibbs Free Energy equation. This highlights that temperature can significantly influence the spontaneity of a reaction.
-
Exothermic reactions (ΔH < 0): These are generally spontaneous (ΔG < 0) at all temperatures because the negative ΔH term dominates.
-
Endothermic reactions (ΔH > 0): These reactions are only spontaneous if the TΔS term is larger than the ΔH term (ΔG < 0). This means that the increase in entropy must be significant enough to overcome the energy barrier imposed by the positive ΔH. This is more likely to occur at high temperatures. For example, melting ice is endothermic but becomes spontaneous above 0°C.
Determining Spontaneity: Practical Applications
Let's look at some practical examples of how to determine spontaneity:
Example 1: Combustion of Methane
The combustion of methane (CH₄) is a highly exothermic reaction (ΔH < 0) that also leads to an increase in entropy (ΔS > 0) due to the formation of gaseous products. Therefore, both enthalpy and entropy favor spontaneity. Consequently, ΔG will be significantly negative, making the reaction highly spontaneous.
Example 2: Formation of Water from Hydrogen and Oxygen
The formation of water from hydrogen and oxygen gas is also exothermic (ΔH < 0). However, the entropy change is negative (ΔS < 0) because two gaseous reactants form a liquid product, which is more ordered. At low temperatures, the negative ΔS term might make the TΔS contribution small, and the reaction could still be spontaneous due to the large negative ΔH. However, at very high temperatures, the TΔS term might become dominant, potentially making the reaction non-spontaneous.
Example 3: Dissolution of Salts
Dissolving some salts in water can be endothermic (ΔH > 0), meaning heat is absorbed from the surroundings. However, the process often involves an increase in entropy (ΔS > 0) because the ions become more dispersed in the solution. At room temperature, the entropy term (TΔS) could outweigh the positive enthalpy term, leading to a negative ΔG and making the dissolution spontaneous.
Beyond Gibbs Free Energy: Other Factors Affecting Spontaneity
While Gibbs Free Energy is a primary indicator of spontaneity, other factors can influence whether a reaction will actually occur:
-
Reaction Kinetics: Even if a reaction is thermodynamically favored (ΔG < 0), it might be kinetically hindered. This means the reaction rate is extremely slow, preventing it from proceeding at a noticeable rate. Activation energy is a major factor here; a high activation energy barrier can prevent a spontaneous reaction from happening at a practical rate. Catalysts can overcome kinetic barriers by lowering the activation energy, thus speeding up the reaction.
-
Reaction Mechanisms: The pathway a reaction follows (its mechanism) can significantly influence its spontaneity. A reaction might be thermodynamically favorable but proceed via a series of steps, some of which might be non-spontaneous, slowing down the overall reaction.
-
Concentration of Reactants and Products: The relative concentrations of reactants and products influence the equilibrium position. Even if ΔG < 0, if the concentration of reactants is very low, the reaction might not proceed noticeably.
-
Presence of Catalysts: Catalysts can accelerate the rate of both forward and reverse reactions without changing the equilibrium constant. They decrease the activation energy, making a spontaneous reaction proceed much faster.
Conclusion: A Holistic View of Spontaneity
Determining if a chemical reaction is spontaneous involves a comprehensive understanding of thermodynamics and kinetics. While Gibbs Free Energy provides a crucial guide, remember that other factors like reaction kinetics, mechanism, concentration, and the presence of catalysts play critical roles in determining whether a thermodynamically favored reaction will actually occur at a perceptible rate. By considering these factors holistically, we can gain a complete picture of the spontaneity of a reaction and its practical implications. The interplay of enthalpy, entropy, and temperature, ultimately expressed through Gibbs Free Energy, is the cornerstone for understanding and predicting the direction of chemical reactions.
Latest Posts
Latest Posts
-
Find The Constant A Such That The Function Is Continuous
Apr 02, 2025
-
How Many Ounces Is 500ml Of Water
Apr 02, 2025
-
What Is The Simplest Form Of 9 12
Apr 02, 2025
-
Write Each Equation In Standard Form Using Integers
Apr 02, 2025
-
What Is 24 In A Fraction
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Do You Know If A Reaction Is Spontaneous . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
