How Are Elements In The Modern Periodic Table Arranged
listenit
Mar 31, 2025 · 7 min read
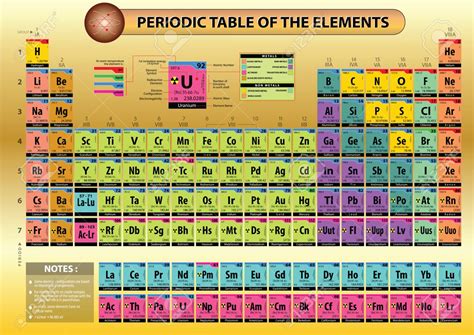
Table of Contents
How Are Elements in the Modern Periodic Table Arranged?
The modern periodic table, a cornerstone of chemistry, is far more than a simple list of elements. It's a meticulously organized arrangement reflecting the fundamental properties and behaviors of atoms, revealing patterns and relationships that underpin our understanding of matter. This intricate organization, however, wasn't achieved overnight. It's the culmination of centuries of scientific inquiry, culminating in a system that elegantly displays the periodic trends and properties of elements. This article delves deep into the principles behind the arrangement of elements in the modern periodic table, exploring the key factors contributing to its structure and the information it reveals about the chemical world.
The Genesis of Organization: Early Attempts at Classification
Before the elegant arrangement we know today, scientists struggled to categorize the growing list of discovered elements. Early attempts were often based on observable physical properties like atomic weight or reactivity. While these attempts were rudimentary, they laid the groundwork for the development of a more comprehensive system.
Döbereiner's Triads (1817):
Johann Wolfgang Döbereiner, a German chemist, observed that certain groups of three elements (triads) shared similar chemical properties and that the atomic weight of the middle element was roughly the average of the other two. Examples included lithium, sodium, and potassium (alkali metals) and chlorine, bromine, and iodine (halogens). While insightful, this system was limited and couldn't accommodate all known elements.
Newlands' Law of Octaves (1864):
John Newlands, a British chemist, proposed the "Law of Octaves," noting that when elements were arranged in order of increasing atomic weight, every eighth element exhibited similar properties, analogous to the octaves in music. This approach, though a significant step forward, faced criticism due to inconsistencies and its inability to account for newly discovered elements.
Mendeleev's Periodic Table (1869):
Dmitri Mendeleev, a Russian chemist, is largely credited with creating the first truly functional periodic table. He arranged elements in order of increasing atomic weight, but crucially, he also grouped elements with similar chemical properties into columns (groups) and arranged them in rows (periods) based on recurring patterns of properties. Mendeleev's genius lay in his bold prediction of the existence and properties of undiscovered elements, leaving gaps in his table to accommodate them. These predictions were later confirmed, solidifying the validity of his periodic system.
The Modern Periodic Table: Arrangement Based on Atomic Number
While Mendeleev's table was remarkably accurate for its time, it wasn't perfect. Discrepancies arose when the atomic weight ordering contradicted the observed chemical properties. The resolution came with the discovery of the atomic nucleus and the concept of atomic number.
Atomic Number as the Organizing Principle:
Henry Moseley's X-ray spectroscopy experiments in the early 20th century revealed that the characteristic X-ray frequencies of elements were directly proportional to their atomic number (the number of protons in the nucleus). This discovery established atomic number as the fundamental organizing principle of the periodic table. Elements are now arranged in order of increasing atomic number, resolving the inconsistencies present in Mendeleev's table based solely on atomic weight.
Periods and Groups:
The modern periodic table is arranged into:
-
Periods (Rows): Each period represents a principal energy level (shell) in an atom. Elements within a period have the same number of electron shells. The number of elements in each period varies, reflecting the number of electrons that can occupy each energy level.
-
Groups (Columns): Each group consists of elements with similar outer electron configurations, resulting in similar chemical properties. These outer electrons, called valence electrons, are primarily responsible for the element's reactivity and bonding behavior. Groups are numbered 1 to 18, with some groups having specific names, like alkali metals (Group 1), alkaline earth metals (Group 2), halogens (Group 17), and noble gases (Group 18).
Blocks:
The periodic table is further divided into blocks based on the subshells in which the outermost electrons reside:
-
s-block: Groups 1 and 2 (alkali and alkaline earth metals). These elements have their valence electrons in the s subshell.
-
p-block: Groups 13 to 18. These elements have their valence electrons in the p subshell. This block includes diverse elements like halogens, noble gases, and metalloids.
-
d-block: Groups 3 to 12 (transition metals). These elements are characterized by the filling of the d subshell. Transition metals exhibit variable oxidation states and form colorful compounds.
-
f-block: Elements placed separately at the bottom of the table (lanthanides and actinides). These elements have their valence electrons in the f subshell. These elements are also known as inner transition metals.
Periodic Trends: Patterns Revealed by the Table's Arrangement
The arrangement of the periodic table elegantly reveals several recurring trends in the properties of elements. These trends are directly related to the variations in effective nuclear charge (the net positive charge experienced by valence electrons) and atomic radius. Understanding these trends is crucial for predicting and explaining the behavior of elements and their compounds.
Atomic Radius:
Atomic radius generally increases down a group (due to the addition of electron shells) and decreases across a period (due to increased effective nuclear charge pulling electrons closer to the nucleus).
Ionization Energy:
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period (due to increased effective nuclear charge) and decreases down a group (due to increased atomic radius and shielding).
Electronegativity:
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period (due to increased effective nuclear charge) and decreases down a group (due to increased atomic radius and shielding).
Electron Affinity:
Electron affinity, the energy change associated with adding an electron to an atom, generally increases across a period and decreases down a group. However, the trend is less regular than ionization energy and electronegativity.
Metallic Character:
Metallic character, which encompasses properties like conductivity and malleability, generally decreases across a period (due to increased ionization energy and electronegativity) and increases down a group (due to decreased ionization energy and electronegativity).
Beyond the Basics: Further Insights from the Periodic Table
The periodic table is not merely a static arrangement; it serves as a dynamic tool providing profound insights into the behavior of elements and their interactions.
Predicting Chemical Reactions:
By understanding the position of an element on the table, we can predict its reactivity and the types of chemical bonds it will form. For instance, elements on the left side of the table (alkali and alkaline earth metals) are highly reactive metals, while those on the right (halogens) are highly reactive nonmetals. The noble gases, in group 18, are largely inert due to their stable electron configurations.
Understanding Chemical Bonding:
The periodic table helps to understand different types of chemical bonding – ionic, covalent, and metallic. Ionic bonds form between elements with significantly different electronegativities (typically a metal and a nonmetal), while covalent bonds form between elements with similar electronegativities (typically nonmetals). Metallic bonds are found within metals, facilitated by the delocalization of valence electrons.
Predicting Physical Properties:
The periodic table also allows for the prediction of certain physical properties, such as melting points, boiling points, and density. These properties often exhibit trends within groups and periods.
Applications in Various Fields:
The periodic table is indispensable across various scientific and technological disciplines:
-
Material Science: Understanding the properties of elements and their alloys is crucial for designing new materials with desired characteristics.
-
Medicine: The periodic table informs the design and development of drugs and medical imaging techniques.
-
Environmental Science: The table is essential for understanding the environmental impact of elements and their compounds.
Conclusion: The Enduring Legacy of the Periodic Table
The modern periodic table, the result of centuries of scientific inquiry and innovation, remains a powerful and versatile tool. Its elegant arrangement reflects the fundamental laws governing the behavior of matter, enabling us to predict, explain, and manipulate the properties of elements and compounds. It's not merely a catalog of elements; it's a window into the very fabric of the chemical universe, providing a framework for understanding the intricacies of the world around us and fueling ongoing advancements in science and technology. The periodic table, far from being a static artifact, is a dynamic, evolving resource, constantly refined and expanded as our understanding of the chemical world deepens.
Latest Posts
Latest Posts
-
Does Transcription Take Place In The Nucleus
Apr 02, 2025
-
What Is 15 As A Fraction
Apr 02, 2025
-
What Is The Lcm For 6 And 10
Apr 02, 2025
-
Which Organelles Supply Energy To The Cell
Apr 02, 2025
-
Why Is Density A Physical Property
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Are Elements In The Modern Periodic Table Arranged . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
