How Are Electrons Arranged In An Atom
listenit
Mar 18, 2025 · 6 min read
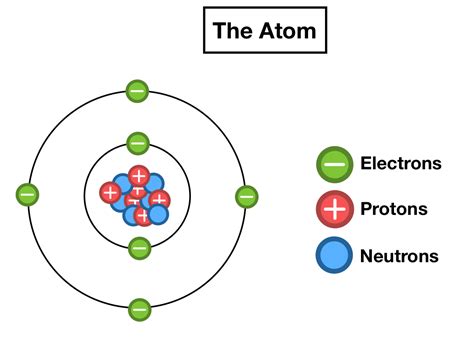
Table of Contents
How Are Electrons Arranged in an Atom? Unveiling the Secrets of Atomic Structure
Understanding the arrangement of electrons within an atom is fundamental to grasping the behavior of matter. This seemingly simple question opens a door to a fascinating world of quantum mechanics, explaining chemical bonding, reactivity, and the properties of elements. This article delves deep into the intricacies of electron arrangement, exploring the historical context, the principles governing electron configuration, and the implications for the periodic table and chemical behavior.
From Bohr's Model to Quantum Mechanics: A Historical Perspective
Early models of the atom struggled to accurately depict electron arrangement. Ernest Rutherford's gold foil experiment revealed the atom's nucleus, but the electron's location remained a mystery. Niels Bohr's model, proposed in 1913, was a significant leap forward. This model depicted electrons orbiting the nucleus in specific energy levels or shells, much like planets orbiting the sun. While simplistic, Bohr's model successfully explained the discrete spectral lines observed in atomic emissions.
However, Bohr's model had limitations. It couldn't accurately predict the spectra of more complex atoms and failed to explain the chemical behavior of elements. The need for a more robust model led to the development of quantum mechanics. This revolutionary theory described electrons not as particles orbiting in definite paths but as probability clouds or orbitals.
The Quantum Mechanical Model: Orbitals and Quantum Numbers
The quantum mechanical model utilizes four quantum numbers to describe the state of an electron within an atom:
1. Principal Quantum Number (n)
The principal quantum number (n) represents the electron shell or energy level. It can take on positive integer values (n = 1, 2, 3,...). Higher values of 'n' indicate higher energy levels and greater distance from the nucleus. Electrons in shells with lower 'n' values are more strongly bound to the nucleus.
2. Azimuthal Quantum Number (l)
The azimuthal quantum number (l) describes the subshell or orbital shape within a shell. For a given value of 'n', 'l' can range from 0 to (n-1). Different values of 'l' correspond to different orbital types:
- l = 0: s orbital (spherical shape)
- l = 1: p orbital (dumbbell shape)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
3. Magnetic Quantum Number (ml)
The magnetic quantum number (ml) specifies the orientation of the orbital in space. For a given value of 'l', ml can range from -l to +l, including 0. For example, a p subshell (l = 1) has three orbitals (ml = -1, 0, +1), oriented along the x, y, and z axes respectively.
4. Spin Quantum Number (ms)
The spin quantum number (ms) describes the intrinsic angular momentum of the electron, often visualized as a spinning motion. It can only take on two values: +1/2 (spin up) or -1/2 (spin down). This is crucial for understanding electron pairing within orbitals.
Electron Configuration and the Aufbau Principle
Electron configuration describes the arrangement of electrons within an atom's orbitals. The Aufbau principle (German for "building-up") dictates that electrons fill the lowest energy levels first. The order of filling is generally:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
This sequence isn't strictly linear due to subtle energy differences between subshells. Hund's rule further specifies that electrons will individually occupy orbitals within a subshell before pairing up. This minimizes electron-electron repulsion. The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons with opposite spins.
Electron Configurations and the Periodic Table
The periodic table's structure is directly related to electron configurations. Elements in the same group (column) have similar outer electron configurations, leading to similar chemical properties. For instance, alkali metals (Group 1) all have a single electron in their outermost s subshell, explaining their high reactivity. Noble gases (Group 18) possess completely filled outer shells, resulting in their inertness. The transition metals have partially filled d subshells, accounting for their variable oxidation states and complex chemistry. The lanthanides and actinides have filling of the f subshells, leading to their unique properties.
Understanding the electron configuration allows us to predict the valency, or the combining capacity of an element. This is critical in understanding how elements form compounds. The number of electrons in the outermost shell (valence electrons) directly influences the number of bonds an atom can form.
Exceptions to the Aufbau Principle
While the Aufbau principle provides a good general guideline, there are exceptions. For some elements, it is energetically favorable for electrons to occupy a higher energy level orbital before completely filling a lower energy level one. These exceptions often occur in transition metals and some post-transition metals. For example, Chromium (Cr) has an electron configuration of [Ar] 3d⁵ 4s¹, instead of the expected [Ar] 3d⁴ 4s². This is due to the extra stability associated with half-filled d subshells. Similarly, Copper (Cu) has [Ar] 3d¹⁰ 4s¹, not [Ar] 3d⁹ 4s². These exceptions highlight the complexity of electron-electron interactions and the limitations of simplified models.
Visualizing Electron Configurations: Orbital Diagrams
Orbital diagrams provide a visual representation of electron configurations. Each orbital is represented by a box, and electrons are represented by arrows, with up arrows indicating spin up and down arrows indicating spin down. This allows for a clear visualization of electron pairing and the occupation of orbitals within subshells. Creating and interpreting orbital diagrams is essential for understanding the behavior of atoms and molecules.
Implications for Chemical Bonding and Reactivity
The arrangement of electrons directly influences an atom's chemical behavior. Atoms tend to react in ways that achieve a stable electron configuration, often resembling that of a noble gas (a filled outer shell). This drives the formation of chemical bonds:
- Ionic bonds: Atoms transfer electrons to achieve stable octets, resulting in the formation of ions and an electrostatic attraction between them.
- Covalent bonds: Atoms share electrons to achieve stable octets, forming molecules.
- Metallic bonds: Electrons are delocalized across a lattice of metal atoms, resulting in the characteristic properties of metals.
Understanding electron configurations is therefore crucial for understanding the nature of chemical bonds and predicting the properties of compounds.
Advanced Concepts: Electron Correlation and Density Functional Theory
The simple model presented above, while useful, does not fully capture the complexity of electron interactions within an atom. Electron correlation describes the influence of electron-electron interactions on electron distribution and energy. Accurate calculations of electron correlation are computationally demanding and require advanced techniques such as density functional theory (DFT). DFT is a powerful computational method that allows for the prediction of various atomic and molecular properties, including electron densities and energy levels.
Conclusion: The Ongoing Exploration of Atomic Structure
The arrangement of electrons in an atom is a captivating area of study. From Bohr's early models to the sophisticated methods of quantum mechanics and DFT, our understanding of atomic structure has evolved dramatically. While the basic principles of electron configuration provide a strong foundation for understanding chemical behavior, ongoing research continues to refine our understanding of electron interactions and refine our predictive capabilities. This exploration is crucial for advancements in materials science, chemistry, and other fields dependent on a deep understanding of the fundamental building blocks of matter. The journey to understanding the intricacies of the atom continues, highlighting the beauty and complexity of the natural world.
Latest Posts
Latest Posts
-
Factor X 2 Y 2 Xy
Mar 18, 2025
-
A Rhombus Is Always A Square
Mar 18, 2025
-
What Is Half Of 1 5 Cup
Mar 18, 2025
-
On The Trip From Detroit To Columbus
Mar 18, 2025
-
Is The Shoulder Distal To The Elbow
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Are Electrons Arranged In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
