Horizontal Rows Of The Periodic Table...
listenit
Mar 24, 2025 · 6 min read
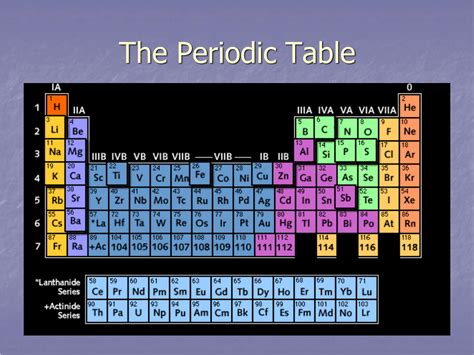
Table of Contents
Delving Deep into the Horizontal Rows of the Periodic Table: Periods and Their Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. While the columns, or groups, represent elements with similar chemical behaviors, the horizontal rows, known as periods, showcase a fascinating trend of changing properties as you move across from left to right. Understanding these periodic trends is crucial for comprehending chemical reactivity, bonding, and the overall behavior of elements. This article delves into the intricacies of the periods, exploring the underlying reasons behind the observed patterns and their implications in various fields of science and technology.
Understanding the Structure of Periods
Each period on the periodic table corresponds to a principal energy level (shell) being filled with electrons. The first period, the shortest, contains only two elements: hydrogen (H) and helium (He). This is because the first energy level (n=1) can only hold a maximum of two electrons. As we move down the table, the periods become longer, reflecting the increasing number of electrons that can be accommodated in successively higher energy levels. The second period accommodates eight electrons, the third eight, and so on, following the pattern dictated by quantum mechanics.
Periodicity and Electron Configuration: The Key Players
The properties of elements within a period are directly linked to their electron configurations. The electronic structure dictates how strongly an atom attracts electrons (electronegativity), how easily it loses electrons (ionization energy), and its overall chemical behavior. As we progress across a period, the number of protons in the nucleus increases, leading to a stronger positive charge. This increased nuclear charge pulls the electrons closer, resulting in observable changes in atomic properties.
Key Periodic Trends Across a Period
Several key properties exhibit systematic changes as we traverse a period:
1. Atomic Radius: A Shrinking Trend
The atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period. This is because the increasing nuclear charge pulls the electrons closer, effectively shrinking the atom's size. While adding electrons to higher energy subshells slightly increases the size (e.g., moving from a 2p orbital to a 3s orbital), the effect of the increased nuclear charge dominates, creating the overall trend of decreasing radius.
2. Ionization Energy: The Energy of Removal
Ionization energy refers to the energy required to remove an electron from a gaseous atom. This energy consistently increases across a period. The stronger nuclear charge holds the electrons more tightly, requiring more energy to overcome the electrostatic attraction and remove an electron. This is particularly true for elements toward the end of the period, as they possess a more complete outermost electron shell.
3. Electronegativity: Electron Attraction
Electronegativity measures the ability of an atom to attract electrons in a chemical bond. It generally increases across a period, mirroring the trend in ionization energy. Atoms with higher electronegativity tend to attract electrons more strongly, leading to the formation of more polar bonds. This property is crucial in determining the nature of chemical bonding and molecular polarity.
4. Electron Affinity: Electron Gain
Electron affinity is the energy change that occurs when an electron is added to a neutral atom. While not showing a completely consistent trend across all periods, generally, electron affinity tends to increase across a period (with some exceptions). Atoms with a high electron affinity are more likely to accept an electron, forming negative ions. The trend is less pronounced than ionization energy or electronegativity, but still reflects the influence of the increasing nuclear charge.
5. Metallic Character: From Metals to Nonmetals
As we move across a period, the metallic character of elements decreases. Metallic character refers to properties such as electrical conductivity, malleability, and ductility. Metals are typically found on the left side of the periodic table, while nonmetals are on the right. This trend arises from the change in how strongly atoms hold onto their electrons. Metals, with their loosely held valence electrons, readily conduct electricity, while nonmetals, with their tightly held electrons, are poor conductors.
6. Oxidation States: Variable Behaviors
The oxidation state of an element represents its apparent charge in a compound. The typical oxidation states across a period reflect the valence electrons available for bonding. For example, alkali metals (Group 1) usually exhibit a +1 oxidation state, while halogens (Group 17) commonly show a -1 oxidation state. However, elements in the transition metal blocks demonstrate a wider range of oxidation states due to the involvement of d-electrons in bonding.
Specific Examples Across Periods
Let's examine a few periods in detail to illustrate these trends:
Period 3 (Sodium to Argon):
- Sodium (Na): Highly reactive metal, low ionization energy, low electronegativity, large atomic radius.
- Magnesium (Mg): Reactive metal, higher ionization energy than Na, slightly higher electronegativity, smaller atomic radius.
- Aluminum (Al): Moderately reactive metal, even higher ionization energy, increasing electronegativity, smaller atomic radius.
- Silicon (Si): Metalloid (intermediate between metal and nonmetal), displays both metallic and nonmetallic properties.
- Phosphorus (P): Nonmetal, higher ionization energy and electronegativity, smaller atomic radius.
- Sulfur (S): Nonmetal, even higher ionization energy and electronegativity, smaller atomic radius.
- Chlorine (Cl): Highly reactive nonmetal, very high ionization energy and electronegativity, small atomic radius.
- Argon (Ar): Inert noble gas, high ionization energy and essentially no electronegativity, small atomic radius.
The progression from highly reactive metal (Na) to inert gas (Ar) beautifully illustrates the periodic trends.
Period 4 (Potassium to Krypton):
Similar trends are observable in Period 4, but with the added complexity of the transition metals (Sc to Zn). The transition metals exhibit variable oxidation states due to the involvement of d-electrons in bonding. The gradual decrease in atomic radius and increase in ionization energy and electronegativity continue across the period. The presence of the transition metals introduces a level of complexity in predicting properties, but the underlying principles remain the same.
Applications and Implications
The understanding of periodic trends has far-reaching implications across various scientific and technological fields:
- Material Science: Designing new materials with specific properties often relies on carefully selecting elements based on their periodic properties. For example, understanding electronegativity differences is crucial for designing semiconductors and other electronic components.
- Catalysis: Catalysts are substances that speed up chemical reactions. The selection of catalysts often relies on understanding the oxidation states and reactivity of different elements.
- Medicine: The periodic table plays a vital role in understanding the interactions of drugs and biological systems. Many essential biological processes involve transition metal ions, and their properties heavily influence their roles.
- Environmental Science: Understanding the chemical behavior of elements is crucial for assessing environmental pollution and developing remediation strategies. The reactivity of elements is crucial in determining their environmental fate and toxicity.
Conclusion: A Continuing Journey of Discovery
The horizontal rows of the periodic table, the periods, provide a framework for understanding the systematic changes in the properties of elements. The interplay of nuclear charge, electron configuration, and electron shielding effects elegantly explains the observed trends in atomic radius, ionization energy, electronegativity, and metallic character. This knowledge is fundamental to various scientific disciplines, paving the way for advancements in materials science, catalysis, medicine, environmental science, and many other fields. As our understanding of atomic structure deepens, further insights into these periodic trends will undoubtedly continue to enrich our knowledge of the chemical world and its vast applications.
Latest Posts
Latest Posts
-
What Fraction Is Equivalent To 5 8
Mar 26, 2025
-
How Does Cell Membrane Help Maintain Homeostasis
Mar 26, 2025
-
How Many Inches In A Quarter Of A Yard
Mar 26, 2025
-
5 To The Power Of Negative 1
Mar 26, 2025
-
What Is 1 8 In A Fraction
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Horizontal Rows Of The Periodic Table... . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
