Heat Of Neutralization Of Hcl And Naoh
listenit
Mar 16, 2025 · 6 min read
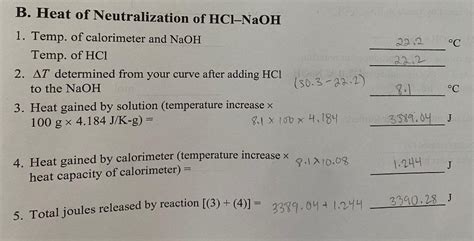
Table of Contents
The Heat of Neutralization of HCl and NaOH: A Comprehensive Guide
The heat of neutralization is a fundamental concept in chemistry, representing the enthalpy change (ΔH) that occurs when an acid and a base react to form a salt and water. This reaction is typically exothermic, meaning it releases heat. This article will delve deep into the heat of neutralization, focusing specifically on the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH), exploring the experimental determination, factors affecting the results, and the theoretical implications.
Understanding the Reaction: HCl + NaOH
The reaction between HCl and NaOH is a classic example of a strong acid-strong base neutralization reaction:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This reaction is highly favorable due to the formation of the stable water molecule and the soluble salt, sodium chloride (NaCl). The heat released during this reaction is primarily attributed to the formation of water molecules from H⁺ and OH⁻ ions. The strong ionic bonds in the reactants are broken, and stronger covalent bonds are formed in the water molecules. This difference in bond energies contributes to the exothermic nature of the reaction.
Experimental Determination of Heat of Neutralization
The heat of neutralization can be experimentally determined using calorimetry. A simple calorimeter, often a polystyrene cup (to minimize heat loss), is used. The process typically involves the following steps:
1. Preparing the Solutions:
- Accurately measure a known volume of a standard HCl solution (e.g., 1.0 M) using a pipette and transfer it to the calorimeter.
- Record the initial temperature of the HCl solution using a thermometer.
- Similarly, measure a known volume of a standard NaOH solution (of the same concentration as the HCl solution to ensure complete neutralization) and record its initial temperature.
2. Mixing and Monitoring Temperature:
- Quickly add the NaOH solution to the calorimeter containing the HCl solution.
- Stir the mixture gently using a thermometer (ensure to minimise heat transfer via the thermometer itself) to ensure uniform temperature distribution.
- Continuously monitor the temperature of the mixture and record the maximum temperature reached.
3. Calculations:
The heat of neutralization can be calculated using the following formula:
ΔH = -mcΔT / n
Where:
- ΔH is the enthalpy change (heat of neutralization) in Joules per mole (J/mol).
- m is the mass of the solution (assuming the density of the solution is approximately 1 g/mL, the mass can be approximated by the volume in mL).
- c is the specific heat capacity of the solution (approximately 4.18 J/g°C for dilute aqueous solutions).
- ΔT is the change in temperature (final temperature - initial temperature) in °C.
- n is the number of moles of water formed (which is equal to the number of moles of either HCl or NaOH, since they react in a 1:1 molar ratio).
Note: The negative sign in the formula indicates that the reaction is exothermic (heat is released).
Sources of Error in Experimental Determination:
Several factors can introduce errors in the experimental determination of the heat of neutralization:
- Heat Loss to Surroundings: Heat can be lost to the surroundings through the calorimeter walls and the thermometer, leading to an underestimate of the true ΔH. This can be minimized by using well-insulated calorimeters and performing the experiment quickly.
- Incomplete Neutralization: If the acid and base are not completely neutralized, the calculated ΔH will be inaccurate. This can be avoided by using stoichiometrically equivalent amounts of acid and base.
- Heat Capacity of the Calorimeter: The calorimeter itself absorbs some of the heat released by the reaction. This can be accounted for by calibrating the calorimeter using a known reaction with a known ΔH.
- Inaccurate Measurements: Inaccurate measurements of volume, temperature, and concentration can lead to significant errors in the calculated ΔH. Care should be taken to use precise measuring instruments and techniques.
Factors Affecting the Heat of Neutralization
The heat of neutralization varies depending on the strength of the acid and base involved. While the neutralization of strong acids and strong bases typically yields a ΔH close to -57 kJ/mol (due to the dominant contribution of water formation), deviations occur with weak acids or weak bases.
Strong Acid-Strong Base Neutralization:
For strong acids like HCl and strong bases like NaOH, the heat of neutralization is relatively constant and close to -57 kJ/mol. This is because the dissociation of strong acids and bases is essentially complete in aqueous solution, leading to a consistent concentration of H⁺ and OH⁻ ions to react and form water.
Weak Acid-Strong Base or Strong Acid-Weak Base Neutralization:
When either the acid or base is weak, the heat of neutralization is less exothermic than -57 kJ/mol. This is because energy is required to ionize the weak acid or weak base before neutralization can occur. This energy input reduces the overall heat released by the reaction. For example, the neutralization of acetic acid (CH₃COOH) with NaOH involves the ionization of acetic acid, requiring energy, and thus resulting in a smaller overall ΔH.
Weak Acid-Weak Base Neutralization:
The heat of neutralization for weak acid-weak base reactions is even less exothermic, reflecting the energy requirements for ionization of both reactants.
Theoretical Implications and Applications
The heat of neutralization has significant theoretical implications and practical applications:
1. Understanding Bond Energies:
The heat of neutralization provides insights into the relative strengths of bonds broken and formed during the reaction. The exothermic nature of strong acid-strong base neutralization reflects the stronger bond formation in water compared to the bonds broken in the reactants.
2. Determining the Strength of Acids and Bases:
The heat of neutralization can be used to determine the strength of acids and bases. Deviations from the typical -57 kJ/mol for strong acid-strong base reactions indicate the presence of weak acids or weak bases.
3. Thermochemistry and Energetics:
The heat of neutralization is a crucial concept in thermochemistry and helps in understanding energy changes in chemical reactions.
4. Industrial Applications:
The concept is relevant in various industrial processes where neutralization reactions are involved, for instance, wastewater treatment and chemical synthesis. Controlling the temperature during neutralization is crucial to ensure efficient and safe operation.
5. Further Research and Studies:
The experimental measurement and theoretical understanding of the heat of neutralization lay the foundation for more advanced studies in solution chemistry and reaction kinetics.
Conclusion
The heat of neutralization of HCl and NaOH is a powerful tool for understanding the energetics of acid-base reactions. The consistent heat released during strong acid-strong base neutralization demonstrates the complete ionization and the significant energy released in water formation. Deviations from this value for other acid-base combinations provide insights into the relative strengths of acids and bases and underscore the influence of ionization energies on the overall enthalpy change. Understanding these concepts is vital for numerous applications across chemistry and related fields. Careful experimental design and precise measurements are crucial for accurate determination of the heat of neutralization. Furthermore, understanding the sources of error and factors influencing the result are essential for interpreting the data correctly and drawing meaningful conclusions. The continuous exploration of this fundamental concept opens doors for deeper insights into the intricacies of chemical reactions and their energetic profiles.
Latest Posts
Latest Posts
-
How Many Radians In A Revolution
Mar 17, 2025
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
-
What Is 10 To The Power Of 7
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Heat Of Neutralization Of Hcl And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
