Five Protons In The Nucleus Of The Atom
listenit
Mar 13, 2025 · 7 min read
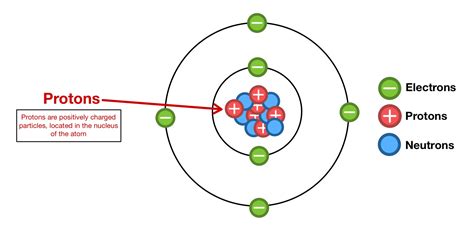
Table of Contents
Five Protons in the Nucleus: Exploring the World of Boron
The seemingly simple concept of an atom, the fundamental building block of matter, holds a universe of complexity within its tiny structure. While we often visualize atoms with a central nucleus and orbiting electrons, the nucleus itself is a fascinating microcosm of particle physics, governed by the strong nuclear force and home to a dynamic dance of protons and neutrons. This article delves into the intriguing world of atoms with five protons in their nucleus – specifically, the element boron – exploring its properties, isotopes, applications, and the unique challenges associated with its nuclear structure.
Understanding the Boron Atom: A Closer Look at Five Protons
Boron, with its atomic number 5, stands out as the only element with a relatively stable isotope containing only five protons in its nucleus. This unique characteristic significantly impacts its chemical and physical properties, setting it apart from its neighbors on the periodic table. Understanding boron's behavior requires a closer look at the interplay between its five protons, its neutrons, and the electrons that surround the nucleus.
The Role of Protons and Neutrons: Stability and Isotopes
The five protons in a boron nucleus determine its atomic number and its fundamental chemical identity. These positively charged particles define boron's place on the periodic table and its interactions with other elements. However, the number of neutrons in the nucleus can vary, leading to different isotopes of boron. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This variation affects the atom's mass and stability.
Boron has two naturally occurring stable isotopes: boron-10 (⁵B) and boron-11 (¹¹B). Boron-10 has five protons and five neutrons, while boron-11 has five protons and six neutrons. The relative abundance of these isotopes influences the average atomic mass of boron, which is approximately 10.81 atomic mass units (amu). The different neutron numbers slightly alter the nuclear properties of these isotopes, affecting their interaction with neutrons in nuclear reactions.
Electron Configuration and Chemical Behavior
The five protons in the boron nucleus attract five electrons, which occupy specific energy levels or orbitals around the nucleus. Boron's electron configuration is 1s²2s²2p¹, meaning two electrons fill the first energy level (1s), two fill the second energy level (2s), and a single electron occupies one of the three 2p orbitals. This single electron in the 2p orbital is responsible for boron's chemical reactivity. It readily participates in chemical bonds, forming covalent bonds with other atoms to achieve a stable electron configuration.
This electron configuration explains boron's behavior as a metalloid, exhibiting properties of both metals and nonmetals. It can act as both an electron donor and an electron acceptor in chemical reactions. This dual nature allows boron to form a variety of compounds with diverse properties.
Isotopes of Boron: A Deeper Dive into Nuclear Variations
The two naturally occurring isotopes of boron, ¹⁰B and ¹¹B, are not just simple variations. Their different neutron numbers lead to significant differences in their nuclear properties, which have important implications for their applications in various fields.
Boron-10: Neutron Capture and Nuclear Applications
Boron-10 possesses a remarkably high cross-section for thermal neutron capture. This means it has a much higher probability of absorbing slow-moving neutrons than most other isotopes. When a boron-10 nucleus absorbs a neutron, it undergoes nuclear fission, producing alpha particles (helium nuclei) and lithium-7 nuclei. This process is highly energetic and forms the basis for several important applications:
-
Neutron detection and shielding: Boron-10's ability to capture neutrons makes it an essential component in neutron detectors and shielding materials in nuclear reactors and other radiation-related applications. The alpha particles produced during neutron capture can be easily detected, providing a means to measure neutron flux. Furthermore, boron-containing materials effectively absorb neutrons, reducing radiation levels.
-
Boron Neutron Capture Therapy (BNCT): This groundbreaking cancer treatment technique leverages boron-10's high neutron capture cross-section. A boron-10-containing drug is selectively delivered to cancerous cells. The patient is then exposed to a beam of neutrons, which are preferentially absorbed by the boron-10 in the tumor cells. The resulting alpha particles cause localized cell death, destroying the cancerous tissue while minimizing damage to surrounding healthy cells. This targeted approach offers a potential advantage over conventional radiation therapies.
Boron-11: The More Abundant Isotope
Boron-11, the more abundant isotope, has a lower neutron capture cross-section than boron-10. While it doesn't exhibit the same remarkable neutron absorption properties, its relatively stable nucleus and natural abundance make it crucial for understanding boron's overall chemical behavior and its applications in various fields. Its stability contributes to the overall average properties observed for boron as an element.
Applications of Boron: From Everyday Uses to Cutting-Edge Technologies
Boron and its compounds find widespread applications in various fields, driven by their unique chemical and physical properties. The versatility of boron stems from its ability to form a variety of compounds, ranging from simple borates to complex organoboron compounds.
Boron in Everyday Life
Boron's presence in our daily lives is often unnoticed. It plays a significant role in several materials and products:
-
Borax and boric acid: These common boron compounds are used in detergents, cleaning agents, insecticides, and flame retardants. Their antiseptic properties make them effective in cleaning and disinfecting.
-
Glass and ceramics: Boron oxide (B₂O₃) is added to glass to improve its durability, thermal resistance, and chemical resistance. It is also used in the production of specialized ceramics and enamels.
-
Agriculture: Boron is an essential micronutrient for plant growth, playing a crucial role in various metabolic processes. Boron deficiency in plants can lead to reduced yields and poor growth. Boron-containing fertilizers are used to supplement boron levels in the soil.
Boron in Advanced Technologies
Beyond everyday applications, boron finds increasing use in advanced technologies:
-
Semiconductors: Boron is a crucial dopant in semiconductor materials, modifying their electrical conductivity. It's used in the production of integrated circuits and other electronic components.
-
Superconductors: Certain boron compounds exhibit superconducting properties, meaning they conduct electricity with zero resistance at low temperatures. Research into these materials is ongoing, with potential applications in energy transmission and other technologies.
-
High-strength materials: Boron fibers are used to reinforce composite materials, creating incredibly strong and lightweight structures used in aerospace, sporting goods, and other applications.
The Challenges and Future of Boron Research
While boron's unique properties offer numerous advantages, research continues to address challenges and explore new possibilities:
Nuclear Structure and Reactivity
The nuclear structure of boron, particularly the interplay between protons and neutrons in its isotopes, remains an active area of research. Understanding the behavior of these isotopes under various conditions, such as high temperatures and pressures, is crucial for optimizing their use in nuclear applications.
Synthesis of Novel Boron Compounds
Scientists are constantly exploring new ways to synthesize novel boron compounds with tailored properties for specific applications. This research involves investigating new reaction pathways and exploring the potential of boron-containing nanomaterials.
Environmental Impact and Sustainability
The increasing use of boron and its compounds necessitates careful consideration of their environmental impact. Research focuses on developing sustainable methods of boron production and use, minimizing potential environmental risks.
Conclusion: A Multifaceted Element with a Bright Future
Boron, with its five protons in the nucleus, represents a fascinating example of an element whose unique properties stem from the delicate interplay of its nuclear and electronic structures. Its diverse applications, ranging from everyday cleaning products to cutting-edge technologies, underscore its importance in modern society. As research continues to unveil new aspects of its behavior and explore new avenues for its application, boron's role in science and technology promises to expand further in the years to come. Its unique nuclear properties, particularly those of boron-10, remain a focal point for advancing nuclear medicine and materials science, solidifying boron’s position as a significant element with a bright future in scientific innovation. The journey of understanding this seemingly simple atom with its five protons continues to yield exciting discoveries, shaping our understanding of the fundamental building blocks of matter and their potential to drive technological progress.
Latest Posts
Latest Posts
-
90 Is 1 10 Of What
Mar 13, 2025
-
What 2 Fractions Are Equivalent To 3 4
Mar 13, 2025
-
Does The Pythagorean Theorem Work On All Triangles
Mar 13, 2025
-
Lowest Common Multiple Of 2 And 8
Mar 13, 2025
-
How Many Square Inches Are In 1 Square Foot
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Five Protons In The Nucleus Of The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
