Enzymes Increase The Rate Of A Reaction By
listenit
Mar 16, 2025 · 6 min read
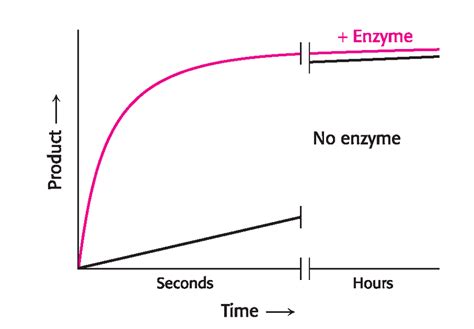
Table of Contents
Enzymes Increase the Rate of a Reaction By: A Deep Dive into Enzyme Kinetics
Enzymes are biological catalysts, remarkable molecules that dramatically accelerate the rate of virtually all chemical reactions within living organisms. Without enzymes, these reactions would proceed far too slowly to sustain life. But how do enzymes achieve this incredible feat? Understanding this involves delving into the intricacies of enzyme kinetics, the study of enzyme-catalyzed reaction rates. This article will explore the mechanisms by which enzymes increase reaction rates, focusing on the key concepts of activation energy, active sites, induced fit, and the factors influencing enzyme activity.
Lowering Activation Energy: The Core Mechanism
At the heart of enzyme catalysis lies the reduction of activation energy (Ea). Activation energy is the minimum energy required for a reaction to occur. Think of it as the energy "hill" that reactants must overcome to transform into products. Reactions with high activation energies proceed slowly because only a small fraction of reactant molecules possess sufficient energy to surpass this barrier.
Enzymes dramatically lower this activation energy barrier, allowing a much larger fraction of reactant molecules to participate in the reaction, thus significantly increasing the reaction rate. They achieve this primarily through several ingenious mechanisms:
1. Substrate Binding and Orientation: The Proximity Effect
Enzymes bind to specific reactant molecules, known as substrates, at their active sites. These active sites are precisely shaped pockets or crevices on the enzyme's surface, exhibiting remarkable specificity. By binding substrates to their active sites, enzymes bring the reactants into close proximity and in the correct orientation. This proximity effect dramatically increases the likelihood of successful collisions between reacting molecules, thus boosting the reaction rate.
Imagine trying to assemble a complex puzzle with pieces scattered across a large table. This is analogous to a reaction without an enzyme – slow and inefficient. An enzyme, on the other hand, acts like a well-organized workbench, holding the puzzle pieces (substrates) in close proximity and the correct orientation, facilitating assembly (reaction).
2. Induced Fit: A Dynamic Embrace
The interaction between an enzyme and its substrate is not static; it's a dynamic process. The induced fit model proposes that the enzyme's active site undergoes a conformational change upon substrate binding. This change optimizes the active site's structure for catalysis, enhancing its ability to lower activation energy. The substrate, in turn, can also undergo conformational changes to better fit within the enzyme's active site.
This dynamic interaction ensures a highly specific and efficient catalytic process. It's like a handshake – the initial contact adjusts to optimize the grip, enabling a stronger and more effective interaction.
3. Strain and Distortion: Destabilizing Reactants
Enzymes can also lower activation energy by straining or distorting the bound substrate molecules. By binding substrates in a strained conformation, enzymes partially break existing bonds within the substrate, making it easier to form new bonds in the transition state. This destabilization of the substrate facilitates the reaction by reducing the energy required to reach the transition state.
4. Acid-Base Catalysis: Proton Transfer
Many enzymes employ acid-base catalysis, using acidic or basic amino acid residues within their active sites to donate or accept protons (H+ ions). This process helps to stabilize the transition state, thereby reducing activation energy. The carefully positioned amino acid residues in the active site act as precise proton donors or acceptors, facilitating the required chemical steps.
5. Covalent Catalysis: Transient Bonds
In covalent catalysis, the enzyme forms a temporary covalent bond with the substrate. This intermediate covalent bond facilitates the reaction by altering the reaction pathway, bypassing the high-energy transition state of the uncatalyzed reaction. Once the reaction is complete, the covalent bond is broken, regenerating the enzyme in its original form.
6. Metal Ion Catalysis: Stabilizing Charges
Metal ions present in the enzyme's active site can play various roles in catalysis. They can participate in redox reactions, stabilize negative charges in transition states, or bridge between the enzyme and the substrate, facilitating efficient catalysis. These metal ions often act as Lewis acids, accepting electron pairs and stabilizing the negatively charged transition states that occur during the reaction.
Factors Affecting Enzyme Activity: A Delicate Balance
The rate of an enzyme-catalyzed reaction is influenced by several factors:
1. Substrate Concentration: The Saturation Effect
At low substrate concentrations, the reaction rate increases proportionally with increasing substrate concentration. This is because more enzyme molecules are able to bind to substrates, leading to more product formation. However, at high substrate concentrations, the reaction rate plateaus. This is known as saturation kinetics, where all enzyme molecules are bound to substrate, and further increases in substrate concentration cannot increase the reaction rate. The maximum rate at saturation is denoted as Vmax.
2. Enzyme Concentration: More Enzymes, More Action
The reaction rate is directly proportional to the concentration of the enzyme. Increasing enzyme concentration increases the number of active sites available for substrate binding, thereby accelerating the reaction.
3. Temperature: Finding the Goldilocks Zone
Enzymes have an optimal temperature at which they function most efficiently. Increasing the temperature initially increases the reaction rate due to increased molecular motion and collision frequency. However, excessively high temperatures can denature the enzyme, causing a loss of its catalytic activity due to structural changes that affect the integrity of the active site.
4. pH: Maintaining the Right Balance
Enzymes also have an optimal pH range at which they function optimally. Deviations from the optimal pH can alter the ionization state of amino acid residues in the active site, affecting substrate binding and catalysis. Extreme pH values can also denature the enzyme.
5. Inhibitors: Interference with Function
Enzyme inhibitors are molecules that reduce enzyme activity. Competitive inhibitors compete with the substrate for binding to the enzyme's active site, while non-competitive inhibitors bind to a different site on the enzyme, altering its conformation and reducing its catalytic efficiency. Inhibitors play crucial roles in regulating metabolic pathways within cells.
6. Activators: Enhancing Performance
Enzyme activators are molecules that enhance enzyme activity. They can bind to the enzyme, inducing a conformational change that increases substrate binding or catalytic efficiency. Activators often play a regulatory role in metabolic pathways, ensuring that enzymes only function when needed.
Enzyme Kinetics: Quantifying Enzyme Activity
The study of enzyme kinetics employs mathematical models to quantify enzyme activity and analyze the factors that influence it. The Michaelis-Menten equation is a fundamental model in enzyme kinetics, describing the relationship between substrate concentration ([S]) and reaction rate (v):
v = Vmax[S] / (Km + [S])
where Vmax is the maximum reaction rate and Km (the Michaelis constant) represents the substrate concentration at which the reaction rate is half of Vmax. Km is a measure of the enzyme's affinity for its substrate – a lower Km indicates a higher affinity.
Conclusion: The Power of Precise Catalysis
Enzymes are master catalysts, increasing the rate of biological reactions by several orders of magnitude. By lowering activation energy through various mechanisms, including substrate binding, induced fit, and strain, they orchestrate the complex chemistry of life. Understanding enzyme kinetics and the factors that modulate enzyme activity is crucial for comprehending cellular processes, developing pharmaceuticals, and engineering novel biocatalysts for industrial applications. The precise, highly regulated catalysis afforded by enzymes underscores their indispensable role in the very fabric of life. Further research continues to unravel the intricate details of enzyme function, revealing ever-more sophisticated strategies for achieving this remarkable acceleration of biochemical reactions.
Latest Posts
Latest Posts
-
3 4 To The Power Of 2
Mar 16, 2025
-
What Is The Lcm Of 10 And 12
Mar 16, 2025
-
What Is The Square Root Of 300
Mar 16, 2025
-
Burning Wood Is A Physical Change
Mar 16, 2025
-
X 2 3 In Radical Form
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Enzymes Increase The Rate Of A Reaction By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
