Enough Of A Monoprotic Acid Is Dissolved In Water
listenit
Mar 15, 2025 · 7 min read
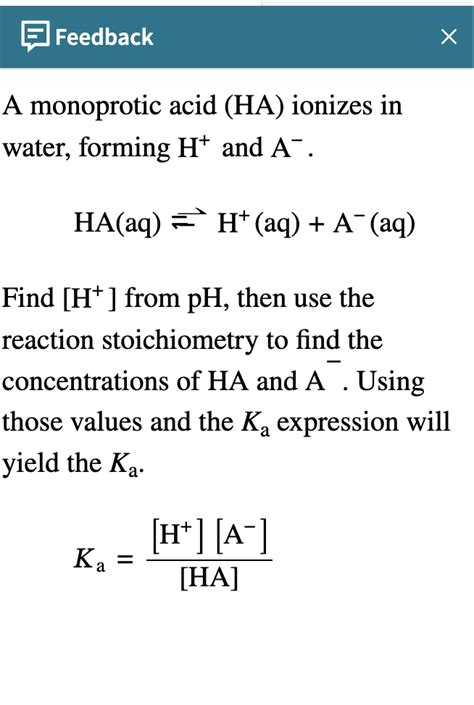
Table of Contents
Enough of a Monoprotic Acid is Dissolved in Water: Understanding Acid Dissociation and Equilibrium
When a monoprotic acid, meaning an acid that can donate only one proton (H⁺) per molecule, is dissolved in water, it undergoes a process called acid dissociation. This process involves the transfer of a proton from the acid molecule to a water molecule, resulting in the formation of hydronium ions (H₃O⁺) and the conjugate base of the acid. Understanding this process is crucial in various fields, including chemistry, environmental science, and biochemistry. This comprehensive article will delve deep into the intricacies of monoprotic acid dissociation, exploring equilibrium constants, calculations, and practical applications.
The Dissociation Process: A Detailed Look
The general equation for the dissociation of a monoprotic acid, HA, in water is:
HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
Where:
- HA represents the monoprotic acid.
- H₂O represents water.
- H₃O⁺ represents the hydronium ion (often simplified to H⁺).
- A⁻ represents the conjugate base of the acid.
The double arrow (⇌) indicates that the reaction is an equilibrium process. This means that the forward reaction (dissociation of the acid) and the reverse reaction (combination of the hydronium ion and the conjugate base to reform the acid) are occurring simultaneously. The relative rates of these reactions determine the position of the equilibrium.
Strong vs. Weak Monoprotic Acids
The extent to which a monoprotic acid dissociates in water is a key characteristic that categorizes it as either a strong acid or a weak acid.
Strong acids completely dissociate in water. This means that essentially all of the acid molecules donate their protons to water molecules. Examples include hydrochloric acid (HCl), nitric acid (HNO₃), and sulfuric acid (H₂SO₄) (although sulfuric acid is diprotic, its first dissociation is essentially complete).
Weak acids, on the other hand, only partially dissociate in water. A significant portion of the acid molecules remain undissociated in solution. The degree of dissociation is much lower than that of strong acids. Examples include acetic acid (CH₃COOH), formic acid (HCOOH), and benzoic acid (C₇H₆O₂).
The Acid Dissociation Constant (Ka)
The position of the equilibrium for the dissociation of a weak acid is quantified by the acid dissociation constant (Ka). Ka is an equilibrium constant that expresses the ratio of the concentrations of products to reactants at equilibrium. For the general dissociation of a monoprotic acid HA:
Ka = [H₃O⁺][A⁻] / [HA]
Where:
- [H₃O⁺] is the concentration of hydronium ions at equilibrium.
- [A⁻] is the concentration of the conjugate base at equilibrium.
- [HA] is the concentration of the undissociated acid at equilibrium.
A larger Ka value indicates a stronger acid, meaning it dissociates more readily and has a higher concentration of H₃O⁺ ions at equilibrium. Conversely, a smaller Ka value indicates a weaker acid.
Calculating Ka and pH
The Ka value can be experimentally determined through various methods, including titration and conductivity measurements. Once the Ka value is known, it can be used to calculate the pH of a solution of a weak acid. The pH is a measure of the acidity or basicity of a solution, defined as the negative logarithm (base 10) of the hydronium ion concentration:
pH = -log₁₀[H₃O⁺]
Calculating the pH of a weak acid solution involves solving an equilibrium expression using the Ka value and the initial concentration of the acid. This often requires the use of the quadratic formula or approximations depending on the relative magnitude of Ka and the initial acid concentration.
The Importance of ICE Tables
When calculating the equilibrium concentrations of species in a weak acid dissociation, an ICE table (Initial, Change, Equilibrium) is a highly useful tool. This table organizes the initial concentration of the acid, the change in concentration due to dissociation, and the equilibrium concentrations of all species.
Let's consider an example: 0.10 M solution of acetic acid (Ka = 1.8 x 10⁻⁵).
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| CH₃COOH | 0.10 | -x | 0.10 - x |
| H₃O⁺ | 0 | +x | x |
| CH₃COO⁻ | 0 | +x | x |
Using the Ka expression:
1.8 x 10⁻⁵ = (x)(x) / (0.10 - x)
Since Ka is very small, we can often approximate (0.10 - x) ≈ 0.10, simplifying the calculation:
1.8 x 10⁻⁵ = x²/0.10
Solving for x (which represents [H₃O⁺]):
x = √(1.8 x 10⁻⁶) = 1.34 x 10⁻³ M
Therefore, the pH of the solution is:
pH = -log₁₀(1.34 x 10⁻³) ≈ 2.87
This demonstrates a typical calculation using an ICE table and the Ka value. However, for acids with larger Ka values or lower initial concentrations, the approximation may not be valid, and the quadratic formula would need to be applied for more accurate results.
Factors Affecting Acid Dissociation
Several factors can influence the extent of dissociation of a monoprotic acid:
-
Temperature: Increasing the temperature generally increases the degree of dissociation for weak acids. This is because the dissociation process is often endothermic (absorbs heat).
-
Concentration: Diluting a weak acid solution increases the degree of dissociation. This is because the equilibrium shifts to the right to compensate for the decrease in concentration of the acid.
-
Common Ion Effect: The presence of a common ion (e.g., the conjugate base) in the solution suppresses the dissociation of the weak acid. This is a direct consequence of Le Chatelier's principle.
Applications of Monoprotic Acid Dissociation
Understanding the principles of monoprotic acid dissociation has wide-ranging applications across numerous scientific and industrial fields:
-
Buffer Solutions: Weak acids and their conjugate bases are crucial components of buffer solutions. These solutions resist changes in pH when small amounts of acid or base are added. This property is vital in biological systems and chemical processes that require a stable pH environment.
-
Titrations: Acid-base titrations rely on the quantitative reaction between an acid and a base to determine the concentration of an unknown solution. Understanding acid dissociation is crucial for interpreting titration curves and accurately calculating concentrations.
-
Environmental Chemistry: The acidity of water bodies is a significant environmental concern. The dissociation of monoprotic acids, such as carbonic acid (H₂CO₃) from dissolved CO₂, plays a crucial role in determining the pH of rain and natural waters. Understanding these processes is essential for managing water quality and addressing acid rain issues.
-
Medicine and Biochemistry: Many biologically important molecules, such as amino acids and organic acids, are monoprotic. Their dissociation properties are crucial for understanding their behavior in biological systems, including enzyme activity and protein structure. Furthermore, many pharmaceutical drugs are weak acids or bases, and their dissociation characteristics are critical for determining their absorption, distribution, metabolism, and excretion (ADME) properties within the body.
-
Industrial Processes: Many industrial processes rely on controlling the pH of solutions. Understanding monoprotic acid dissociation is critical for optimizing these processes and ensuring product quality and efficiency.
Advanced Concepts and Further Exploration
This article has provided a foundational understanding of monoprotic acid dissociation. For a more in-depth analysis, further exploration into the following areas is recommended:
-
Polyprotic Acids: These acids can donate more than one proton per molecule. Their dissociation involves multiple equilibrium constants and requires a more complex approach to calculations.
-
Acid-Base Theories: Brønsted-Lowry and Lewis acid-base theories provide a more comprehensive framework for understanding acid-base reactions beyond the simple proton transfer model.
-
Thermodynamics of Acid Dissociation: The thermodynamic aspects of acid dissociation, including enthalpy and entropy changes, offer a deeper understanding of the driving forces behind these reactions.
-
Spectroscopic Techniques: Techniques such as UV-Vis and NMR spectroscopy can be used to directly monitor the dissociation process and determine the concentrations of different species at equilibrium.
By grasping the fundamental concepts of monoprotic acid dissociation, you build a strong foundation for understanding more complex acid-base chemistry and its diverse applications across various scientific disciplines. This knowledge empowers you to solve intricate problems, interpret experimental data, and contribute to advancements in related fields.
Latest Posts
Latest Posts
-
Does Period Come Before Or After Quotes
May 09, 2025
-
What Was The Religion Of Virginia Colony
May 09, 2025
-
Express Your Answer As A Condensed Structural Formula
May 09, 2025
-
How Many Ounce In A Half Gallon
May 09, 2025
-
Square Root Of 42 In Radical Form
May 09, 2025
Related Post
Thank you for visiting our website which covers about Enough Of A Monoprotic Acid Is Dissolved In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.