Does Nh3 Have A Dipole Moment
listenit
Mar 22, 2025 · 6 min read
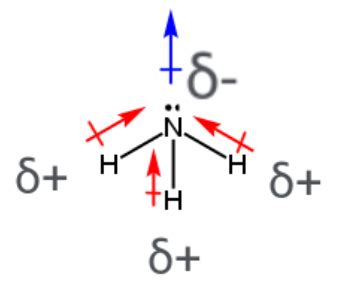
Table of Contents
Does NH₃ Have a Dipole Moment? A Deep Dive into Ammonia's Polarity
Ammonia (NH₃), a ubiquitous compound in nature and industry, presents a fascinating case study in molecular polarity. Understanding whether or not it possesses a dipole moment is crucial for grasping its chemical behavior and properties. This in-depth exploration will delve into the intricacies of ammonia's structure, bonding, and the resultant dipole moment.
Understanding Dipole Moments
Before examining ammonia specifically, let's establish a foundational understanding of dipole moments. A dipole moment is a measure of the separation of positive and negative charges within a molecule. It arises when there's an uneven distribution of electron density, resulting in one end of the molecule carrying a slightly positive charge (δ+) and the other end carrying a slightly negative charge (δ-). This separation is represented by a vector quantity, with the magnitude indicating the strength of the dipole and the direction pointing from the positive to the negative pole.
Several factors influence the presence and magnitude of a dipole moment:
-
Bond Polarity: A polar bond exists when there's a significant difference in electronegativity between the atoms involved. Electronegativity reflects an atom's ability to attract electrons in a chemical bond. The greater the electronegativity difference, the more polar the bond.
-
Molecular Geometry: Even if individual bonds are polar, the overall molecular geometry can influence whether the dipole moments of individual bonds cancel each other out. Symmetrical molecules often have zero net dipole moments, even if they contain polar bonds. Conversely, asymmetrical molecules generally possess a net dipole moment.
The Structure of Ammonia (NH₃)
Ammonia's molecular structure is crucial in determining its dipole moment. The central nitrogen atom is bonded to three hydrogen atoms via single covalent bonds. Nitrogen is more electronegative than hydrogen (3.0 vs 2.1 on the Pauling scale). This electronegativity difference creates polar N-H bonds, with the nitrogen atom carrying a partial negative charge (δ-) and the hydrogen atoms carrying partial positive charges (δ+).
However, the story doesn't end there. Ammonia doesn't adopt a planar structure; instead, it exhibits a trigonal pyramidal geometry. This is due to the presence of a lone pair of electrons on the nitrogen atom. This lone pair occupies a significant amount of space and repels the bonding pairs of electrons, pushing the three N-H bonds downwards and resulting in the pyramidal shape.
The Impact of Molecular Geometry on Dipole Moment in NH₃
The trigonal pyramidal geometry is the key to understanding why ammonia possesses a dipole moment. If ammonia were planar (like boron trifluoride, BF₃), the individual bond dipole moments would cancel each other out, resulting in a zero net dipole moment. However, the pyramidal shape prevents this cancellation.
The bond dipoles of the three N-H bonds are not symmetrically arranged around the nitrogen atom. They are directed downwards towards the nitrogen atom, and are partially countered by the contribution from the lone pair of electrons pushing in the opposite direction. The net effect is a non-zero dipole moment. The lone pair contributes significantly to the overall molecular dipole, making it stronger than if the molecule were only considering the N-H bonds.
Visualizing the Dipole Moment
Imagine three arrows representing the bond dipoles, pointing from the partially positive hydrogen atoms towards the partially negative nitrogen atom. These arrows do not cancel each other out because of the pyramidal shape. Instead, they combine vectorially, resulting in a resultant dipole moment pointing from the approximate center of the three hydrogen atoms towards the nitrogen atom, which includes the effect of the lone pair.
Quantifying the Dipole Moment of NH₃
The dipole moment of ammonia is experimentally determined to be approximately 1.47 Debye (D). This value reflects the significant separation of charge within the molecule. This relatively high value indicates a substantial degree of polarity, which greatly influences ammonia's physical and chemical properties.
Consequences of Ammonia's Dipole Moment
The presence of a dipole moment has several important consequences for ammonia's behavior:
-
Solubility: Ammonia is highly soluble in polar solvents like water because of the strong dipole-dipole interactions between the ammonia molecules and the water molecules. The partial positive charges on the hydrogen atoms of ammonia are attracted to the partially negative oxygen atoms of water, and vice versa.
-
Boiling Point: Ammonia has a higher boiling point than other molecules of comparable molecular weight that lack dipole moments. The stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding) arising from the dipole moment require more energy to overcome during the phase transition from liquid to gas.
-
Hydrogen Bonding: The presence of a dipole moment and the lone pair of electrons on nitrogen enable ammonia to participate in hydrogen bonding. Hydrogen bonding is a particularly strong type of dipole-dipole interaction, contributing significantly to ammonia's high boiling point and other properties.
-
Reactivity: The polarity of the N-H bonds in ammonia influences its reactivity. The partial negative charge on the nitrogen makes it susceptible to electrophilic attack, while the partial positive charge on the hydrogen atoms makes it prone to nucleophilic attack.
Comparing NH₃ with Other Molecules
It's instructive to compare ammonia with other molecules to further emphasize the role of geometry in determining dipole moment:
-
Carbon Tetrachloride (CCl₄): Although the C-Cl bonds are polar, the tetrahedral geometry of CCl₄ results in the cancellation of individual bond dipoles, leading to a zero net dipole moment. This makes CCl₄ a nonpolar molecule despite containing polar bonds.
-
Water (H₂O): Like ammonia, water has a bent molecular geometry due to the presence of lone pairs on the oxygen atom. This asymmetry leads to a significant dipole moment, making water a highly polar molecule. The dipole moment of water is higher than that of ammonia, primarily due to the greater electronegativity difference between oxygen and hydrogen compared to nitrogen and hydrogen.
Conclusion
In conclusion, ammonia (NH₃) unequivocally possesses a dipole moment. This is a direct consequence of the polar N-H bonds and the molecule's trigonal pyramidal geometry, which prevents the cancellation of individual bond dipoles. The resulting polarity significantly influences ammonia's physical and chemical properties, including its solubility, boiling point, hydrogen bonding capabilities, and reactivity. Understanding the relationship between molecular structure, bond polarity, and dipole moment is crucial for predicting and explaining the behavior of various chemical compounds. The case of ammonia serves as a powerful illustration of this fundamental concept in chemistry. The non-zero dipole moment of NH₃ is a direct result of its asymmetric molecular geometry and the difference in electronegativity between nitrogen and hydrogen, making it a polar molecule with a significant impact on its diverse chemical interactions.
Latest Posts
Latest Posts
-
What Is The Fraction Of 65
Mar 24, 2025
-
Is Light Wave A Transverse Wave
Mar 24, 2025
-
What Is 95 Pounds In Kg
Mar 24, 2025
-
What Is The Thinnest Layer Of The Earth Called
Mar 24, 2025
-
What Is The Square Root Of 0 25
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Does Nh3 Have A Dipole Moment . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
