Does Electronegativity Decrease Down A Group
listenit
Mar 22, 2025 · 5 min read
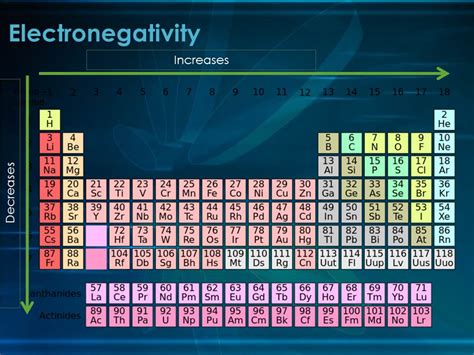
Table of Contents
Does Electronegativity Decrease Down a Group? A Comprehensive Exploration
Electronegativity, a fundamental concept in chemistry, describes an atom's ability to attract electrons towards itself within a chemical bond. Understanding how electronegativity changes within the periodic table is crucial for predicting molecular polarity, bond type, and overall chemical behavior. This article delves into the question: Does electronegativity decrease down a group? The answer is a resounding yes, but understanding why requires a deeper exploration of atomic structure and bonding.
The Periodic Trend: Electronegativity and Group Number
The periodic table is a powerful tool for predicting chemical properties. Elements are organized by atomic number, reflecting the number of protons in their nucleus. This organization reveals periodic trends, including electronegativity. As we move down a group (a vertical column in the periodic table), electronegativity generally decreases. This is a crucial trend to understand for predicting the behavior of compounds formed by elements within the same group.
Why Electronegativity Decreases Down a Group
The decrease in electronegativity down a group is primarily due to two factors:
-
Increased Atomic Radius: As you move down a group, the number of electron shells increases. This leads to a larger atomic radius. The increased distance between the nucleus (positively charged) and the valence electrons (negatively charged) weakens the attractive force between them. Consequently, the nucleus has less pull on the electrons involved in bonding, leading to reduced electronegativity. Think of it like this: the further away the electrons are, the weaker the grip the nucleus has on them.
-
Shielding Effect: The addition of inner electron shells (those between the nucleus and the valence electrons) creates a shielding effect. These inner electrons partially block the positive charge of the nucleus from reaching the valence electrons. This shielding effect reduces the effective nuclear charge experienced by the valence electrons, further diminishing the atom's ability to attract electrons in a bond. The more inner shells, the stronger the shielding, and the lower the electronegativity.
Illustrative Examples Across Groups
Let's examine specific examples to illustrate this trend:
Group 1: Alkali Metals
Consider Group 1, the alkali metals (Li, Na, K, Rb, Cs, Fr). Lithium (Li) has the highest electronegativity in this group. As we move down, the electronegativity decreases progressively: Li > Na > K > Rb > Cs > Fr. This is consistent with the increase in atomic radius and shielding effect. Francium, the heaviest alkali metal, has the lowest electronegativity.
Group 17: Halogens
Group 17, the halogens (F, Cl, Br, I, At), provides another compelling example. Fluorine (F) is the most electronegative element in the entire periodic table. As we progress down the group to chlorine (Cl), bromine (Br), iodine (I), and astatine (At), the electronegativity steadily decreases. The increased distance between the nucleus and valence electrons, combined with enhanced shielding, explains this trend.
Group 14: Carbon Group
Even in groups with more complex electronic configurations like Group 14 (C, Si, Ge, Sn, Pb), the electronegativity trend persists. Carbon exhibits higher electronegativity than silicon, which in turn is more electronegative than germanium, and so on. The underlying reasons remain the same: increasing atomic radius and increased shielding leading to weaker nuclear attraction for bonding electrons.
Exceptions and Nuances
While the general trend of decreasing electronegativity down a group is well-established, there can be subtle exceptions or deviations. These exceptions are usually small and don't significantly alter the overall trend. They may arise due to complexities in electron-electron repulsions or slight variations in effective nuclear charge. However, these variations are minor compared to the dominant effect of increasing atomic radius and shielding.
Implications of Electronegativity Trends
Understanding the decrease in electronegativity down a group has significant implications across various aspects of chemistry:
-
Bond Polarity: The difference in electronegativity between two bonded atoms determines the polarity of the bond. A large electronegativity difference results in a polar bond, while a small difference leads to a nonpolar bond. Knowing the electronegativity trend helps predict the polarity of bonds in compounds.
-
Bond Type: Electronegativity plays a role in determining the type of bond formed between atoms. Large electronegativity differences often lead to ionic bonds (transfer of electrons), while smaller differences result in covalent bonds (sharing of electrons).
-
Molecular Geometry and Properties: The polarity of individual bonds, determined by electronegativity differences, influences the overall polarity and geometry of molecules, impacting their physical and chemical properties, such as melting point, boiling point, and solubility.
-
Reactivity: Electronegativity influences the reactivity of elements and compounds. Elements with high electronegativity tend to be more reactive, readily accepting electrons to achieve a stable electronic configuration.
Beyond Electronegativity: Other Periodic Trends
It's crucial to remember that electronegativity is just one of several periodic trends. Others, closely related, include:
- Atomic Radius: As mentioned, this increases down a group due to the addition of electron shells.
- Ionization Energy: This is the energy required to remove an electron from an atom. It generally decreases down a group due to increased atomic radius and shielding.
- Electron Affinity: This is the energy change associated with adding an electron to an atom. Its trend down a group is more complex and less consistent than electronegativity or ionization energy.
Conclusion: A Consistent Trend with Significant Implications
In summary, electronegativity consistently decreases down a group in the periodic table. This fundamental trend arises from the increasing atomic radius and the shielding effect of inner electrons, which weaken the attractive force of the nucleus on valence electrons. Understanding this trend is crucial for predicting and interpreting various chemical properties, including bond polarity, bond type, molecular geometry, and reactivity. While minor exceptions might exist, the overall pattern remains robust and serves as a powerful tool for comprehending the behavior of elements and their compounds. The implications of this trend extend throughout the vast landscape of chemistry, influencing numerous fields of study and application.
Latest Posts
Latest Posts
-
What Is 5 Percent Of 600
Mar 23, 2025
-
Is Orange Juice A Homogeneous Mixture
Mar 23, 2025
-
The Two Dna Strands Are Held Together By
Mar 23, 2025
-
Solving Systems Of Equations By Substitution Answer Key
Mar 23, 2025
-
What Is 5 7 As A Decimal
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Does Electronegativity Decrease Down A Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
