Does Co2 Have Dipole Dipole Forces
listenit
Mar 19, 2025 · 5 min read
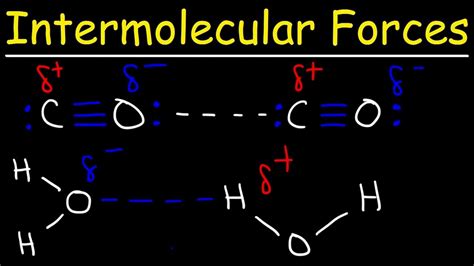
Table of Contents
Does CO2 Have Dipole-Dipole Forces? Understanding Molecular Polarity and Intermolecular Forces
Carbon dioxide (CO₂) is a ubiquitous molecule found in the Earth's atmosphere, playing a crucial role in the planet's climate and the carbon cycle. Understanding its properties, especially its intermolecular forces, is essential for comprehending its behavior in various systems. A common question that arises is: Does CO₂ have dipole-dipole forces? The answer, surprisingly, is no. This article delves into the intricacies of molecular polarity and intermolecular forces to explain why.
Understanding Molecular Polarity
Before addressing the question directly, we need to establish a solid understanding of molecular polarity. A molecule's polarity is determined by the electronegativity difference between its constituent atoms and the molecule's geometry.
Electronegativity: The Tug-of-War of Electrons
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Atoms with higher electronegativity values pull electrons more strongly. When atoms with different electronegativities bond, the electrons are not shared equally, resulting in a polar bond. This unequal sharing creates a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom.
Molecular Geometry: The Shape of the Matter
The shape of a molecule, determined by its electron geometry and molecular geometry (VSEPR theory), plays a critical role in determining its overall polarity. Even if a molecule contains polar bonds, its overall polarity can be zero if the polar bonds cancel each other out due to the molecule's symmetrical shape.
CO₂'s Linear Structure and Nonpolar Nature
Carbon dioxide (CO₂) has a linear molecular geometry. A carbon atom is double-bonded to two oxygen atoms, arranged in a straight line (O=C=O). While the C=O bonds are polar (oxygen is more electronegative than carbon), the symmetry of the molecule leads to the cancellation of these bond dipoles. The dipole moment vectors of the two C=O bonds are equal in magnitude but point in opposite directions, resulting in a net dipole moment of zero.
Visualizing the Cancellation of Dipoles
Imagine two vectors, each representing the dipole moment of a C=O bond. Because the molecule is linear, these vectors are directly opposite each other. When you add these vectors, they cancel each other out, leaving a net dipole moment of zero. This is crucial because the presence or absence of a net dipole moment directly impacts the types of intermolecular forces a molecule can exhibit.
Intermolecular Forces: The Glue That Holds Molecules Together
Intermolecular forces are the attractive forces between molecules. These forces are significantly weaker than the intramolecular forces (bonds) that hold atoms within a molecule together, yet they are crucial for determining a substance's physical properties, such as boiling point, melting point, and solubility. The strength of these forces directly correlates with the molecule's polarity. The primary types of intermolecular forces include:
- Dipole-dipole forces: These forces exist between polar molecules. The partially positive end of one molecule attracts the partially negative end of another molecule.
- London Dispersion Forces (LDFs): These forces are present in all molecules, regardless of polarity. They arise from temporary, instantaneous fluctuations in electron distribution, creating temporary dipoles that induce dipoles in neighboring molecules.
- Hydrogen bonding: This is a special type of dipole-dipole force that occurs when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and is attracted to another highly electronegative atom in a nearby molecule.
Why CO₂ Doesn't Exhibit Dipole-Dipole Forces
Given that CO₂ has a net dipole moment of zero, it cannot exhibit dipole-dipole forces. The symmetrical arrangement of the polar bonds cancels out the individual bond dipoles, eliminating the necessary condition for dipole-dipole interactions.
The Dominance of London Dispersion Forces in CO₂
Since CO₂ is nonpolar, the primary intermolecular forces it experiences are London Dispersion Forces (LDFs). These forces are relatively weak compared to dipole-dipole forces or hydrogen bonding. However, their cumulative effect is significant, especially in larger CO₂ molecules. The size of the CO2 molecule and the number of electrons influence the strength of LDFs.
Comparing CO₂ with Other Molecules
To further illustrate the concept, let's compare CO₂ with a polar molecule like water (H₂O). Water has a bent molecular geometry, and despite the relatively small electronegativity difference between hydrogen and oxygen, the asymmetrical shape prevents the bond dipoles from canceling each other out. This results in a significant net dipole moment, allowing water molecules to exhibit strong dipole-dipole forces and hydrogen bonding, leading to its high boiling point and other unique properties.
Implications of CO₂'s Intermolecular Forces
The fact that CO₂ primarily interacts through weak London Dispersion Forces has significant implications for its physical properties and its behavior in the environment. Its relatively low boiling point (-78.5°C) is a direct consequence of these weak intermolecular forces. This low boiling point is a key factor in the gaseous nature of CO₂ at room temperature and pressure.
CO₂'s Role in Climate Change
The weak intermolecular forces of CO₂ also influence its ability to absorb and radiate infrared radiation, contributing to the greenhouse effect. While the specific mechanisms are complex, the relatively weak interactions between CO₂ molecules affect its radiative properties and its interaction with other greenhouse gases in the atmosphere.
Conclusion: A Nonpolar Molecule with Weak Intermolecular Forces
In conclusion, CO₂ does not have dipole-dipole forces due to its linear molecular geometry and the cancellation of its bond dipoles. This results in a net dipole moment of zero. The predominant intermolecular forces in CO₂ are London Dispersion Forces, which are relatively weak. Understanding the interplay between molecular polarity, intermolecular forces, and the specific characteristics of CO₂ is crucial for comprehending its behavior in various chemical and physical contexts, including its significant role in the Earth's climate system. The absence of dipole-dipole forces, therefore, is a fundamental characteristic defining many of its properties. This knowledge helps us understand not just the molecule itself, but its role in larger systems and processes.
Latest Posts
Latest Posts
-
What Element Has The Lowest Electronegativity
Mar 20, 2025
-
Which Layer Of The Atmosphere Does Weather Occur In
Mar 20, 2025
-
The Si Unit For Power Is The
Mar 20, 2025
-
How Much Is 1 4 Lb
Mar 20, 2025
-
How Many Quarts In 40 Gallons
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Does Co2 Have Dipole Dipole Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
