Do Strong Acids Dissociate In Water
listenit
Apr 07, 2025 · 6 min read
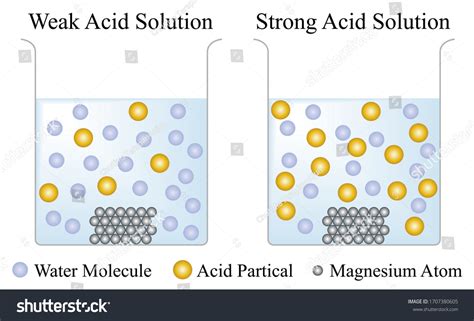
Table of Contents
Do Strong Acids Dissociate in Water? A Comprehensive Exploration
Strong acids are notorious for their potent reactivity, a characteristic deeply rooted in their complete dissociation in water. Understanding this dissociation is crucial for comprehending their behavior in various chemical reactions and applications. This article delves into the intricacies of strong acid dissociation, exploring the underlying mechanisms, factors influencing the process, and its implications in diverse scientific fields.
What are Strong Acids?
Before delving into the dissociation process, let's establish a clear definition. Strong acids are chemical compounds that readily and completely donate a proton (H⁺ ion) to water when dissolved. This complete ionization is the hallmark of a strong acid, differentiating it from its weaker counterparts. This complete donation leads to a high concentration of hydronium ions (H₃O⁺) in the solution, resulting in a significantly low pH.
Examples of Strong Acids:
Several common acids fall under the strong acid category. Some prominent examples include:
- Hydrochloric acid (HCl): A highly corrosive acid found in gastric juice and used extensively in industrial processes.
- Sulfuric acid (H₂SO₄): A viscous, highly corrosive acid used in the production of fertilizers, detergents, and other chemicals. Note that it dissociates in two steps, with the first step being essentially complete.
- Nitric acid (HNO₃): A strong oxidizing acid used in the production of fertilizers, explosives, and other chemicals.
- Hydrobromic acid (HBr): A highly corrosive acid used in various chemical syntheses.
- Hydroiodic acid (HI): Another highly corrosive acid employed in several chemical applications.
- Perchloric acid (HClO₄): A highly reactive and potentially explosive acid often used in analytical chemistry.
The Dissociation Process: A Detailed Look
The dissociation of a strong acid in water is a virtually irreversible process. When a strong acid molecule is introduced to water, it readily donates its proton to a water molecule, forming a hydronium ion (H₃O⁺) and an anion. This process can be represented with a simple equation:
HA(aq) + H₂O(l) → H₃O⁺(aq) + A⁻(aq)
Where:
- HA represents the strong acid molecule.
- H₂O represents water.
- H₃O⁺ represents the hydronium ion.
- A⁻ represents the conjugate base of the acid.
This equation highlights the complete transfer of the proton from the acid to the water molecule. The equilibrium lies heavily towards the right, indicating that almost all the acid molecules have dissociated. This contrasts sharply with weak acids, where the equilibrium lies significantly towards the left, signifying incomplete dissociation.
The Role of Water:
Water acts as a Brønsted-Lowry base in this reaction, accepting the proton from the strong acid. Its polar nature facilitates the interaction with the acid molecule, enabling the proton transfer. The oxygen atom in water, being slightly negative, attracts the positively charged proton, weakening the bond within the acid molecule and facilitating its dissociation.
The Formation of Hydronium Ions:
The formation of hydronium ions (H₃O⁺) is a crucial aspect of strong acid dissociation. These ions are responsible for the characteristic properties of acidic solutions, such as their low pH and their ability to react with bases. The concentration of hydronium ions directly reflects the strength of the acid; a stronger acid will produce a higher concentration of hydronium ions.
Factors Influencing Dissociation:
While strong acids exhibit complete dissociation in water, several factors can subtly influence the extent of dissociation:
- Concentration: Although strong acids dissociate completely, at extremely high concentrations, the activity of the ions might be affected by interionic forces, slightly reducing the effective concentration of H₃O⁺ ions. However, at typical concentrations used in laboratories and industrial settings, this effect is negligible.
- Temperature: Temperature can influence the rate of dissociation. Increased temperature generally accelerates the dissociation process, although the extent of dissociation remains essentially complete for strong acids.
- Solvent: While water is the most common solvent, the dissociation of a strong acid can be affected by the solvent's polarity and ability to stabilize the ions formed. Using non-polar solvents will drastically reduce the dissociation.
Implications of Strong Acid Dissociation:
The complete dissociation of strong acids has profound implications across diverse fields:
1. Industrial Applications:
Strong acids play vital roles in numerous industrial processes. Their complete dissociation provides a readily available source of protons for catalyzing reactions, facilitating various chemical transformations. This is crucial in areas such as:
- Chemical synthesis: Strong acids are indispensable catalysts and reagents in many chemical reactions, including esterification, dehydration, and isomerization.
- Petroleum refining: Strong acids are used in various processes within petroleum refining, including alkylation and isomerization.
- Metal processing: Strong acids are employed in the processing of metals, including pickling (removal of oxides from metal surfaces).
2. Analytical Chemistry:
Strong acids are essential tools in analytical chemistry. Their complete dissociation allows for precise control over pH, which is critical for various analytical techniques:
- Titrations: Strong acids are frequently used as titrants in acid-base titrations to determine the concentration of unknown solutions.
- pH measurements: Strong acids are employed in the preparation of buffer solutions and standard solutions for pH calibration.
3. Biological Systems:
Although less common in this context compared to the industrial and analytical scenarios, controlled use of strong acids is crucial in some biological systems and studies:
- Digestion: Hydrochloric acid (HCl) in the stomach plays a crucial role in the digestion of food.
4. Environmental Concerns:
The highly reactive nature of strong acids necessitates careful handling and disposal to minimize environmental impact. Acid rain, for instance, a result of atmospheric pollutants reacting with water to form strong acids, poses significant environmental concerns.
Strong Acids vs. Weak Acids: A Comparison
Understanding the difference between strong and weak acids is crucial. While both donate protons, their behavior differs significantly due to the extent of dissociation:
| Feature | Strong Acid | Weak Acid |
|---|---|---|
| Dissociation | Complete in water | Partial in water |
| Equilibrium | Lies far to the right (products favored) | Lies far to the left (reactants favored) |
| Hydronium Ions | High concentration | Low concentration |
| pH | Significantly low | Relatively higher |
| Conductivity | High electrical conductivity | Low electrical conductivity |
| Examples | HCl, H₂SO₄, HNO₃, HBr, HI, HClO₄ | CH₃COOH (acetic acid), H₂CO₃ (carbonic acid) |
Conclusion:
The complete dissociation of strong acids in water is a fundamental concept in chemistry with far-reaching implications. This characteristic defines their reactivity, influences their applications in various fields, and underscores the importance of safe handling and disposal. Understanding the mechanisms behind this dissociation, the factors influencing it, and the resulting consequences is essential for anyone working with acids or studying chemical processes. Further research into the nuances of strong acid behavior, particularly under extreme conditions, continues to contribute to advancements across various scientific disciplines.
Latest Posts
Latest Posts
-
Does A Trapezoid Have 4 Right Angles
Apr 08, 2025
-
The Central Part Of An Atom Containing Protons And Neutrons
Apr 08, 2025
-
Position The Following Items In Order Of Decreasing Size
Apr 08, 2025
-
Whats The Square Root Of 108
Apr 08, 2025
-
What Are The Two Most Common Elements In Earths Crust
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Do Strong Acids Dissociate In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.