Do Polar Substances Dissolve In Water
listenit
Apr 01, 2025 · 6 min read
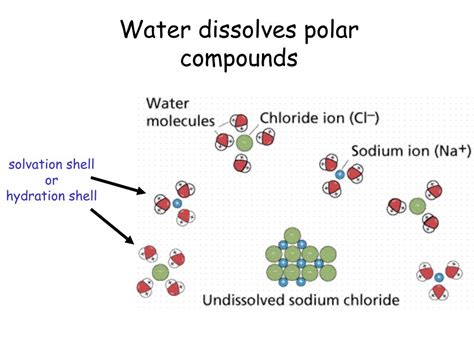
Table of Contents
Do Polar Substances Dissolve in Water? A Deep Dive into Solubility
The question of whether polar substances dissolve in water is fundamental to understanding chemistry and its applications in various fields. The simple answer is: yes, polar substances generally dissolve well in water. But the "why" behind this statement is far more intricate and fascinating. This article will delve into the detailed mechanisms governing the solubility of polar substances in water, exploring the concepts of polarity, hydrogen bonding, and the role of intermolecular forces. We’ll also examine exceptions to this rule and discuss the practical implications of this phenomenon.
Understanding Polarity: The Key to Solubility
Polarity refers to the distribution of electrical charge within a molecule. A molecule is considered polar if it possesses a dipole moment, meaning there's an uneven distribution of electrons, creating a slightly positive end (δ+) and a slightly negative end (δ-). This occurs when there's a significant difference in electronegativity between the atoms within the molecule. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond.
Water (H₂O) is the quintessential example of a polar molecule. The oxygen atom is significantly more electronegative than the hydrogen atoms, pulling the shared electrons closer to itself. This results in a partial negative charge on the oxygen and partial positive charges on the hydrogens. This polarity is responsible for many of water's unique properties.
The Role of Hydrogen Bonding
The strong attraction between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another water molecule is known as hydrogen bonding. Hydrogen bonds are a special type of dipole-dipole interaction, significantly stronger than typical dipole-dipole forces. This extensive hydrogen bonding network contributes significantly to water's high boiling point, surface tension, and its exceptional ability to dissolve polar substances.
How Polar Substances Dissolve in Water: A Microscopic View
The dissolution of a polar substance in water is a dynamic process driven by the interplay of intermolecular forces. When a polar substance is added to water, the polar molecules of the substance interact with the polar water molecules. The slightly positive ends of the solute molecules are attracted to the slightly negative oxygen atoms of water, while the slightly negative ends of the solute molecules are attracted to the slightly positive hydrogen atoms of water.
This attraction overcomes the attractive forces holding the solute molecules together (like dipole-dipole interactions or hydrogen bonds within the solute itself). The solute molecules become surrounded by water molecules, a process called solvation or hydration in the case of water. This process continues until the solute is completely dispersed in the water, forming a homogeneous solution.
Think of it like this: imagine trying to mix sugar (a polar substance) into water. The sugar molecules are initially clustered together. But when you add them to water, the polar water molecules wedge themselves between the sugar molecules, breaking apart the sugar crystal and surrounding individual sugar molecules. The strong attractions between the water and sugar molecules keep the sugar dissolved.
Examples of Polar Substances That Dissolve in Water
Many everyday substances are polar and readily dissolve in water. These include:
- Sugars: Sucrose (table sugar), glucose, fructose are all polar molecules due to the presence of many hydroxyl (-OH) groups which create significant dipole moments.
- Alcohols: Ethanol (drinking alcohol), methanol, propanol all contain hydroxyl groups, making them polar and soluble in water. However, as the length of the carbon chain increases (in higher alcohols), the nonpolar character dominates, reducing water solubility.
- Acids: Acetic acid (vinegar), citric acid (found in citrus fruits) are polar due to the presence of carboxyl (-COOH) groups, making them readily soluble in water.
- Salts: Sodium chloride (table salt), potassium chloride, magnesium sulfate – ionic compounds, dissolve in water because the ions (cations and anions) are strongly attracted to the polar water molecules. The positive ions are surrounded by the negative oxygen ends of water, while the negative ions are surrounded by the positive hydrogen ends. This process is called dissociation.
- Many amino acids and proteins: Amino acids, the building blocks of proteins, often contain polar functional groups (like carboxyl and amino groups) making them soluble in water. The solubility of proteins depends on their overall structure and the distribution of polar and nonpolar amino acid residues.
Exceptions and Limitations: When Polarity Doesn't Guarantee Solubility
While polarity is a major factor in determining solubility, it's not the only one. Several factors can influence the solubility of polar substances in water:
- Molecular Size and Shape: Very large polar molecules may have reduced solubility despite their polarity. The size can hinder the effective interaction of the solute molecule with water molecules. Similarly, an awkwardly shaped molecule may not fit well within the water’s hydrogen-bonded network.
- Presence of Nonpolar Regions: Polar molecules with significant nonpolar regions (hydrophobic regions) may exhibit limited solubility in water. The hydrophobic regions tend to cluster together, minimizing contact with water, and leading to lower overall solubility. This is often seen in larger organic molecules like fatty acids, where a long hydrocarbon chain (nonpolar) is attached to a polar carboxyl group.
- Temperature: Temperature affects the kinetic energy of molecules. Increasing the temperature generally increases solubility because it provides more energy to overcome intermolecular forces.
- Pressure: Pressure has a relatively minor effect on the solubility of liquids and solids in water. However, for gases, increasing pressure increases solubility.
The Importance of "Like Dissolves Like"
The principle of "like dissolves like" is a fundamental guideline in predicting solubility. It emphasizes that substances with similar polarities tend to dissolve in each other. Polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes. Water, being a polar solvent, is excellent at dissolving polar and ionic substances. Conversely, nonpolar solvents, such as hexane or benzene, readily dissolve nonpolar solutes like oils and fats.
Practical Applications of Polar Substance Solubility
The solubility of polar substances in water has numerous practical applications across various disciplines:
- Biology: Water's ability to dissolve polar molecules is crucial for life. Biological processes rely heavily on aqueous solutions, where polar molecules like sugars, amino acids, and ions are transported and react.
- Medicine: Many drugs are designed to be polar or to ionize in water to facilitate their absorption into the bloodstream. Water-based solutions are commonly used for administering medications.
- Chemistry: Water is a ubiquitous solvent in chemical reactions, facilitating the mixing of reactants and the separation of products.
- Environmental Science: Understanding the solubility of pollutants in water is crucial for assessing environmental risks and developing effective remediation strategies.
- Food Science: Solubility is critical in food processing and formulation. The dissolution of various ingredients in water contributes to texture, flavor, and stability of food products.
Conclusion: A Complex but Crucial Concept
The solubility of polar substances in water is a multifaceted phenomenon governed by the interplay of intermolecular forces, polarity, hydrogen bonding, and other factors like molecular size and shape. While the general rule is that polar substances dissolve well in water, exceptions exist, highlighting the complexity of this fundamental chemical concept. Understanding these principles is essential across numerous scientific disciplines and practical applications, from understanding biological processes to developing new medicines and tackling environmental challenges. This understanding of "like dissolves like" serves as a valuable tool in predicting and manipulating solubility for various purposes.
Latest Posts
Latest Posts
-
How Big Is A Star Compared To Earth
Apr 02, 2025
-
24 To The Power Of 2
Apr 02, 2025
-
Lowest Common Multiple Of 14 And 35
Apr 02, 2025
-
What Is The Limit Of A Constant
Apr 02, 2025
-
X 3 2x 2 X 2 0
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Do Polar Substances Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
