Do Gases Have A Definite Shape
listenit
Mar 17, 2025 · 5 min read
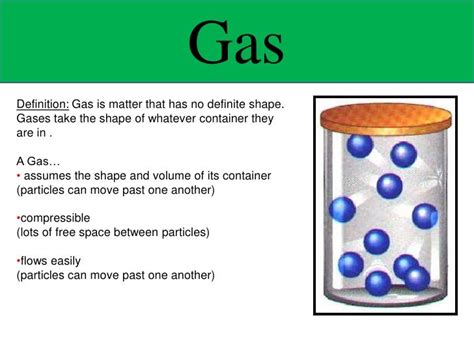
Table of Contents
- Do Gases Have A Definite Shape
- Table of Contents
- Do Gases Have a Definite Shape? Exploring the Properties of Gases
- The Kinetic Molecular Theory: The Foundation of Gas Behavior
- Why Gases Don't Have a Definite Shape: The Role of Intermolecular Forces
- Comparing Gases to Solids and Liquids: A Shape Perspective
- The Compressibility of Gases: A Consequence of Indefinite Shape
- Real Gases vs. Ideal Gases: Deviations from the Ideal Model
- Applications and Examples of Gaseous Indefinite Shape
- Conclusion: The Indefinite Shape – A Defining Characteristic of Gases
- Latest Posts
- Latest Posts
- Related Post
Do Gases Have a Definite Shape? Exploring the Properties of Gases
Gases are one of the four fundamental states of matter, alongside solids, liquids, and plasmas. Understanding their properties, particularly their lack of a definite shape, is crucial to grasping the behavior of matter at a fundamental level. This article delves deep into the characteristics of gases, explaining why they don't possess a fixed shape and exploring the implications of this property in various contexts.
The Kinetic Molecular Theory: The Foundation of Gas Behavior
The kinetic molecular theory (KMT) provides the fundamental framework for understanding the behavior of gases. This theory rests on several key postulates:
- Gases are composed of tiny particles: These particles can be atoms or molecules, depending on the gas in question. Their size is negligible compared to the distances between them.
- These particles are in constant, random motion: They are constantly moving in straight lines until they collide with each other or the container walls.
- Collisions are elastic: This means that no kinetic energy is lost during collisions. The total kinetic energy of the system remains constant.
- There are no significant attractive or repulsive forces between gas particles: The particles are essentially independent of each other, except during collisions.
- The average kinetic energy of the particles is directly proportional to the absolute temperature: Higher temperatures mean faster-moving particles.
This constant, random motion is the key to understanding why gases don't have a definite shape. Because the particles are not bound to fixed positions, they readily move to fill any available space.
Why Gases Don't Have a Definite Shape: The Role of Intermolecular Forces
Unlike solids, where particles are held tightly together in a fixed arrangement, and liquids, where particles are close but can move past each other, gas particles are far apart and experience negligible intermolecular forces. These weak attractive forces, such as van der Waals forces, are simply overcome by the high kinetic energy of the rapidly moving particles.
This lack of significant intermolecular attraction is what allows gas particles to spread out and occupy the entire volume of their container. They are not bound to a specific location or arrangement, leading to their indefinite shape. They simply conform to the shape of whatever container they are in.
Comparing Gases to Solids and Liquids: A Shape Perspective
To further illustrate this concept, let's compare the shape of gases to that of solids and liquids:
- Solids: Solids have a definite shape and volume. The strong intermolecular forces hold their particles in a fixed, rigid structure. The shape remains constant regardless of the container.
- Liquids: Liquids have a definite volume but an indefinite shape. While the intermolecular forces are weaker than in solids, they are still strong enough to keep the particles relatively close together. Liquids take the shape of their container but maintain a constant volume.
- Gases: Gases have neither a definite shape nor a definite volume. The weak intermolecular forces and high kinetic energy of the particles allow them to expand to fill any available space. They readily adopt the shape of their container.
The Compressibility of Gases: A Consequence of Indefinite Shape
The indefinite shape of gases also leads to another important property: compressibility. Because the particles are widely spaced, it's relatively easy to push them closer together, reducing the volume of the gas. This is in stark contrast to solids and liquids, where the particles are already close together, making compression much more difficult.
This compressibility is exploited in various applications, such as compressed air tanks for scuba diving or pneumatic tools. The ability to store a large volume of gas in a smaller space is a direct consequence of the gas's indefinite shape and the large intermolecular distances.
Real Gases vs. Ideal Gases: Deviations from the Ideal Model
The kinetic molecular theory describes ideal gases, which are theoretical gases that perfectly follow the postulates of the KMT. However, real gases deviate from ideal behavior, particularly at high pressures and low temperatures.
At high pressures, the gas particles are forced closer together, and the intermolecular forces become more significant. These forces can cause the gas to deviate from its predicted volume and pressure, making it less compressible than an ideal gas.
At low temperatures, the kinetic energy of the particles decreases, making the intermolecular forces more influential. This can lead to condensation, where the gas transitions into a liquid state, completely losing its indefinite shape.
Despite these deviations, the KMT still provides a valuable framework for understanding the behavior of real gases under many conditions, particularly at moderate temperatures and pressures.
Applications and Examples of Gaseous Indefinite Shape
The indefinite shape of gases has widespread implications across various fields:
- Weather Patterns: The movement and distribution of gases in the atmosphere, influenced by temperature and pressure gradients, drive weather patterns. The gases expand and contract, changing their volume and impacting atmospheric pressure.
- Respiration: The process of breathing relies on the expansion and contraction of our lungs to intake and expel gases like oxygen and carbon dioxide.
- Inflation: Filling balloons, tires, and other inflatable objects utilizes the ability of gases to expand and conform to the shape of the container.
- Aerosols: Spray cans and other aerosol products rely on the ability of gases to propel liquids or solids out of a container.
- Industrial Processes: Many industrial processes utilize gases as reactants or solvents, taking advantage of their ability to fill reaction vessels and mix readily.
- Diffusion and Effusion: Gases exhibit the properties of diffusion (spreading out) and effusion (escaping through small openings), both consequences of their constant random motion and lack of a defined shape.
Conclusion: The Indefinite Shape – A Defining Characteristic of Gases
In conclusion, the indefinite shape of gases is a direct consequence of their unique molecular structure and behavior. The postulates of the kinetic molecular theory, particularly the negligible intermolecular forces and constant random motion of particles, explain why gases readily expand to fill any available space and conform to the shape of their container. While real gases deviate from ideal behavior under certain conditions, the KMT provides a valuable model for understanding their behavior and the widespread implications of their indefinite shape in various scientific and technological applications. This property is fundamental to understanding not only the behavior of gases themselves but also the broader world around us, influencing phenomena from weather patterns to the functionality of everyday objects. Further exploration of this characteristic is critical in advancing fields ranging from materials science and atmospheric modeling to medicine and engineering.
Latest Posts
Latest Posts
-
What Is 1 20 As A Percentage
Mar 18, 2025
-
Chromosomes Are Duplicated During What Stage Of The Cell Cycle
Mar 18, 2025
-
During Which Phases Of Cellular Respiration Is Co2 Produced
Mar 18, 2025
-
The Level With The Most Energy Is The Level
Mar 18, 2025
-
How Many Oz Is 500 Ml Of Water
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Do Gases Have A Definite Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
