Do Acids Lose Or Gain Hydrogen Ions
listenit
Mar 18, 2025 · 6 min read
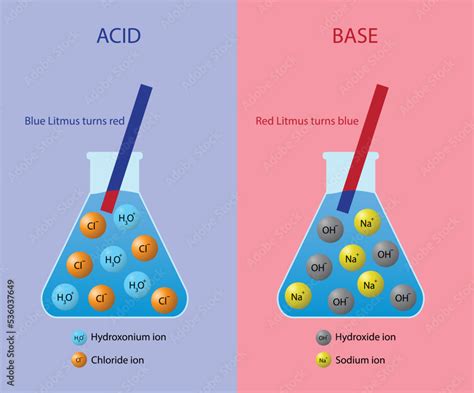
Table of Contents
Do Acids Lose or Gain Hydrogen Ions? Understanding Acid-Base Chemistry
Acids and bases are fundamental concepts in chemistry, playing crucial roles in numerous natural processes and industrial applications. Understanding their behavior, particularly concerning hydrogen ions (H⁺), is essential for grasping many chemical reactions and phenomena. This article delves deep into the question: do acids lose or gain hydrogen ions? We will explore the Brønsted-Lowry acid-base theory, delve into the properties of acids, and examine various examples to solidify our understanding. We'll also touch upon the practical implications of acid-base reactions.
The Brønsted-Lowry Theory: The Foundation of Acid-Base Behavior
The Brønsted-Lowry theory provides a comprehensive framework for understanding acid-base reactions. This theory defines an acid as a substance that donates a proton (H⁺), and a base as a substance that accepts a proton. This definition contrasts with the older Arrhenius theory which only considered acids as substances producing H⁺ ions in aqueous solutions. The Brønsted-Lowry theory encompasses a broader range of substances and reactions.
Crucially, this theory emphasizes the transfer of protons. Acids, therefore, lose a hydrogen ion (proton), while bases gain a hydrogen ion. This proton transfer is the defining characteristic of a Brønsted-Lowry acid-base reaction. The process is often represented by the following generalized equation:
HA + B⁻ ⇌ A⁻ + HB
Where:
- HA is the acid (donates a proton)
- B⁻ is the base (accepts a proton)
- A⁻ is the conjugate base of HA (what remains after HA loses a proton)
- HB is the conjugate acid of B⁻ (what is formed after B⁻ gains a proton)
Acids: Proton Donors – The Key to Understanding Ion Loss
The core characteristic of an acid, according to the Brønsted-Lowry definition, is its ability to donate a proton. This donation process involves the loss of a positively charged hydrogen ion (H⁺). This loss of a proton results in the formation of a conjugate base, which is the species remaining after the acid has donated its proton.
Strong acids readily and completely donate their protons in aqueous solutions. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃). These acids essentially completely dissociate into their ions in water:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
Weak acids, on the other hand, only partially donate their protons. This means that an equilibrium is established between the undissociated acid and its ions. Acetic acid (CH₃COOH) is a classic example:
CH₃COOH(aq) ⇌ H⁺(aq) + CH₃COO⁻(aq)
The equilibrium lies significantly to the left, indicating that most of the acetic acid molecules remain undissociated. However, even weak acids still lose protons, albeit to a lesser extent than strong acids.
Examples Illustrating Proton Loss in Acids
Let's examine a few specific examples to reinforce the concept of acids losing hydrogen ions:
1. Hydrochloric Acid (HCl) and Water
When hydrochloric acid is dissolved in water, it completely dissociates, demonstrating the complete loss of a proton:
HCl(aq) + H₂O(l) → H₃O⁺(aq) + Cl⁻(aq)
Here, HCl acts as the acid, donating a proton to water (which acts as a base). The resulting hydronium ion (H₃O⁺) represents the protonated water molecule. The chloride ion (Cl⁻) is the conjugate base of HCl. This reaction clearly shows the loss of a hydrogen ion from HCl.
2. Acetic Acid (CH₃COOH) and Water
The reaction of acetic acid with water showcases the behavior of a weak acid:
CH₃COOH(aq) + H₂O(l) ⇌ H₃O⁺(aq) + CH₃COO⁻(aq)
This reaction reaches an equilibrium, with only a portion of the acetic acid molecules donating a proton. Nevertheless, the proton donation, and thus the loss of a hydrogen ion, is evident. The acetate ion (CH₃COO⁻) is the conjugate base.
3. Carbonic Acid (H₂CO₃) and Water
Carbonic acid, formed when carbon dioxide dissolves in water, is another example of a weak acid:
H₂CO₃(aq) + H₂O(l) ⇌ H₃O⁺(aq) + HCO₃⁻(aq)
Again, the equilibrium lies to the left, but the reaction clearly demonstrates the loss of a proton from carbonic acid, forming the bicarbonate ion (HCO₃⁻) as the conjugate base.
The Role of Conjugate Bases
The concept of a conjugate base is inextricably linked to the loss of a hydrogen ion by an acid. When an acid loses a proton, the remaining species is its conjugate base. This conjugate base is what is left after the proton donation process is complete. The conjugate base can, in fact, accept a proton again (acting as a base) in a reversible reaction, showing the dynamic nature of acid-base reactions.
Beyond Aqueous Solutions: Acid Behavior in Other Solvents
While the above examples focus on aqueous solutions, the principle of acids losing protons applies in other solvents as well. In non-aqueous solvents, the proton might be transferred to a different solvent molecule or another base present in the solution. The fundamental principle remains the same: acids donate protons.
Practical Implications of Acid-Base Reactions and Proton Transfer
The loss of hydrogen ions by acids has wide-ranging practical implications across various fields:
-
Industrial Processes: Acid-base reactions are crucial in many industrial processes, including the production of fertilizers, pharmaceuticals, and plastics. The controlled addition of acids or bases is used to adjust pH levels and drive specific reactions.
-
Environmental Chemistry: Understanding acid-base chemistry is vital for analyzing environmental issues such as acid rain and soil acidity. The impact of acid deposition on ecosystems is largely determined by the proton donation by acidic compounds.
-
Biological Systems: Biological systems rely heavily on acid-base reactions to maintain homeostasis. The pH of blood, for example, is tightly regulated through buffering systems that involve proton donation and acceptance. Enzymes, essential for biological processes, often function optimally within specific pH ranges.
-
Analytical Chemistry: Acid-base titrations are a fundamental technique in analytical chemistry used to determine the concentration of unknown acids or bases. These titrations involve the controlled addition of an acid or base to determine the endpoint where the reaction is complete.
Conclusion: Acids Unequivocally Lose Hydrogen Ions
In conclusion, the Brønsted-Lowry theory unambiguously defines acids as proton donors. This means that acids lose hydrogen ions (protons) in acid-base reactions. This proton transfer is a fundamental characteristic of acidic behavior, whether in strong or weak acids, and irrespective of the solvent. The loss of hydrogen ions drives numerous chemical reactions with significant implications across diverse scientific and industrial domains. A firm grasp of this principle is critical for understanding various chemical processes and phenomena. Further exploration of acid-base chemistry reveals the complex and fascinating interplay of protons and their impact on the world around us.
Latest Posts
Latest Posts
-
Is Rotting A Physical Or Chemical Change
Mar 18, 2025
-
Which One Warm The Solvent Decrease
Mar 18, 2025
-
What Is The Least Common Multiple Of 2 And 12
Mar 18, 2025
-
Simplify 1 X 1 1 X
Mar 18, 2025
-
5 Meters Is How Many Centimeters
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Do Acids Lose Or Gain Hydrogen Ions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
