Difference Between Molecular Orbital Theory And Valence Bond Theory
listenit
Mar 28, 2025 · 6 min read
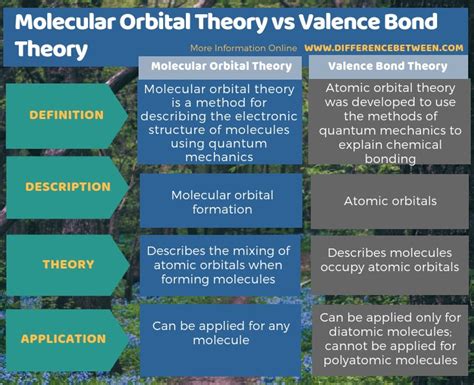
Table of Contents
Delving Deep: Molecular Orbital Theory vs. Valence Bond Theory
Understanding the behavior of molecules is fundamental to chemistry. Two prominent theories, Molecular Orbital (MO) Theory and Valence Bond (VB) Theory, offer distinct yet complementary perspectives on chemical bonding and molecular structure. While both aim to describe how atoms combine to form molecules, they differ significantly in their approaches and the resulting descriptions. This in-depth analysis will explore these differences, highlighting their strengths and weaknesses and showcasing when each theory proves particularly useful.
The Core Differences: A Bird's Eye View
At their heart, MO and VB theory differ in their fundamental assumptions about electron behavior within a molecule.
-
Valence Bond Theory (VB): VB theory assumes that electrons maintain their atomic orbital identities within a molecule. Bond formation is described as the overlap of atomic orbitals from different atoms, creating a region of high electron density between the nuclei. This overlap leads to a stabilizing interaction, forming a chemical bond.
-
Molecular Orbital Theory (MO): MO theory takes a more holistic approach. It postulates that when atoms combine, their atomic orbitals combine to form new molecular orbitals that encompass the entire molecule. Electrons are then distributed among these molecular orbitals, some bonding (lower in energy) and some antibonding (higher in energy).
This seemingly subtle difference in approach leads to significant consequences in how we describe bonding, predict molecular properties, and interpret experimental data.
Valence Bond Theory: A Localized Picture
VB theory provides a relatively intuitive picture of bonding. It's based on the concept of atomic orbitals combining to form localized bonds.
Key Concepts in VB Theory:
-
Atomic Orbital Overlap: The strength of a bond is directly related to the extent of overlap between the atomic orbitals. Greater overlap leads to a stronger bond. The geometry of the molecule is determined by the orientation of these overlapping orbitals.
-
Hybridization: To account for the observed geometries of molecules (e.g., the tetrahedral geometry of methane), VB theory introduces the concept of hybridization. This involves mixing atomic orbitals within a single atom to create new hybrid orbitals that are optimally oriented for bonding. Examples include sp, sp², and sp³ hybridization.
-
Resonance: Many molecules cannot be adequately described by a single Lewis structure. VB theory addresses this using the concept of resonance, where the true structure is a weighted average of multiple contributing Lewis structures. For example, benzene is represented by two resonance structures to capture the delocalization of electrons.
-
Limitations of VB Theory: While conceptually simple, VB theory struggles with molecules possessing significant electron delocalization, such as conjugated systems or aromatic compounds. It also faces challenges in accurately predicting the properties of excited states and radicals.
Molecular Orbital Theory: A Delocalized Perspective
MO theory offers a more sophisticated and mathematically rigorous description of molecular structure and bonding.
Key Concepts in MO Theory:
-
Linear Combination of Atomic Orbitals (LCAO): The foundation of MO theory is the LCAO approximation. Atomic orbitals from different atoms are mathematically combined to generate molecular orbitals that span the entire molecule. The number of molecular orbitals formed equals the number of atomic orbitals combined.
-
Bonding and Antibonding Orbitals: The combination of atomic orbitals leads to the formation of both bonding and antibonding molecular orbitals. Bonding orbitals are lower in energy than the original atomic orbitals and concentrate electron density between the nuclei, strengthening the bond. Antibonding orbitals are higher in energy and have nodes (regions of zero electron density) between the nuclei, weakening the bond.
-
Electron Configuration: Electrons are then filled into the molecular orbitals according to the Aufbau principle and Hund's rule, just like in atomic orbital filling. The electron configuration dictates the bond order and overall stability of the molecule.
-
Delocalization: MO theory naturally accounts for electron delocalization in molecules with conjugated systems or aromatic rings. Electrons are not confined to specific bonds but are distributed across the entire molecule, leading to enhanced stability.
-
Strengths and Weaknesses of MO Theory: MO theory provides a more accurate description of molecular properties, particularly for molecules with significant electron delocalization. It is also more versatile in handling excited states and radicals. However, it can be more complex computationally and less intuitive than VB theory.
Head-to-Head Comparison: A Detailed Analysis
| Feature | Valence Bond Theory | Molecular Orbital Theory |
|---|---|---|
| Basic Idea | Overlap of atomic orbitals | Combination of atomic orbitals into molecular orbitals |
| Bonding | Localized bonds | Delocalized bonds |
| Electron Location | Localized to specific bonds | Delocalized across the molecule |
| Geometry | Determined by orbital hybridization | Determined by the number and type of molecular orbitals |
| Electron Configuration | Implicit | Explicit, filled molecular orbitals |
| Resonance | Uses resonance structures to describe delocalization | Naturally accounts for delocalization |
| Computational Complexity | Relatively simpler | More complex |
| Intuitiveness | More intuitive | Less intuitive |
| Accuracy | Less accurate for delocalized electrons | More accurate for delocalized electrons |
| Excited States | Less accurate | More accurate |
Application and Choice of Theory
The choice between VB and MO theory depends on the specific application and the type of molecule under consideration.
-
VB Theory is preferred when:
- A simple, intuitive picture of bonding is sufficient.
- The molecule has predominantly localized bonds.
- Qualitative understanding of bond angles and geometries is needed.
-
MO Theory is preferred when:
- Accurate prediction of molecular properties is required.
- The molecule has significant electron delocalization.
- Understanding excited states or the behavior of radicals is necessary.
- Quantitative analysis of bonding is needed.
Beyond the Basics: Advanced Concepts
Both VB and MO theories have been refined and extended over time to address their limitations and enhance their predictive power.
Advanced VB Theory:
-
Configuration Interaction (CI): CI goes beyond simple resonance structures and considers the mixing of different electronic configurations to improve the accuracy of energy calculations.
-
Multiconfigurational Self-Consistent Field (MCSCF): MCSCF methods allow for the optimization of multiple electronic configurations simultaneously.
Advanced MO Theory:
-
Density Functional Theory (DFT): DFT is a powerful computational method that focuses on the electron density rather than explicitly calculating wavefunctions. It's widely used for large molecules and complex systems.
-
Post-Hartree-Fock Methods: These methods build upon the basic Hartree-Fock MO approach by incorporating electron correlation effects to improve the accuracy of calculations. Examples include Møller-Plesset perturbation theory (MP2) and coupled-cluster theory (CCSD).
Conclusion: A Complementary Partnership
MO and VB theories, despite their differences, are not mutually exclusive. They offer complementary perspectives on molecular bonding. VB theory provides a conceptually simple and often intuitive understanding of localized bonds, while MO theory offers a more rigorous and accurate description of delocalized electrons. The best approach often depends on the system being studied and the specific information sought. In many cases, a combined approach, leveraging the strengths of both theories, can provide the most complete and insightful understanding of molecular behavior. The continued development and refinement of both VB and MO theories ensure their continued relevance and importance in modern chemical research.
Latest Posts
Latest Posts
-
Can A Standard Deviation Be Negative
Mar 31, 2025
-
80 Is What Percent Of 40
Mar 31, 2025
-
Is The Square Root Of 5 A Rational Number
Mar 31, 2025
-
Graph 4x 7 Greater Than X 13
Mar 31, 2025
-
8 Quarts Equals How Many Pints
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Molecular Orbital Theory And Valence Bond Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
