Difference Between Formula Mass And Molar Mass
listenit
May 10, 2025 · 6 min read
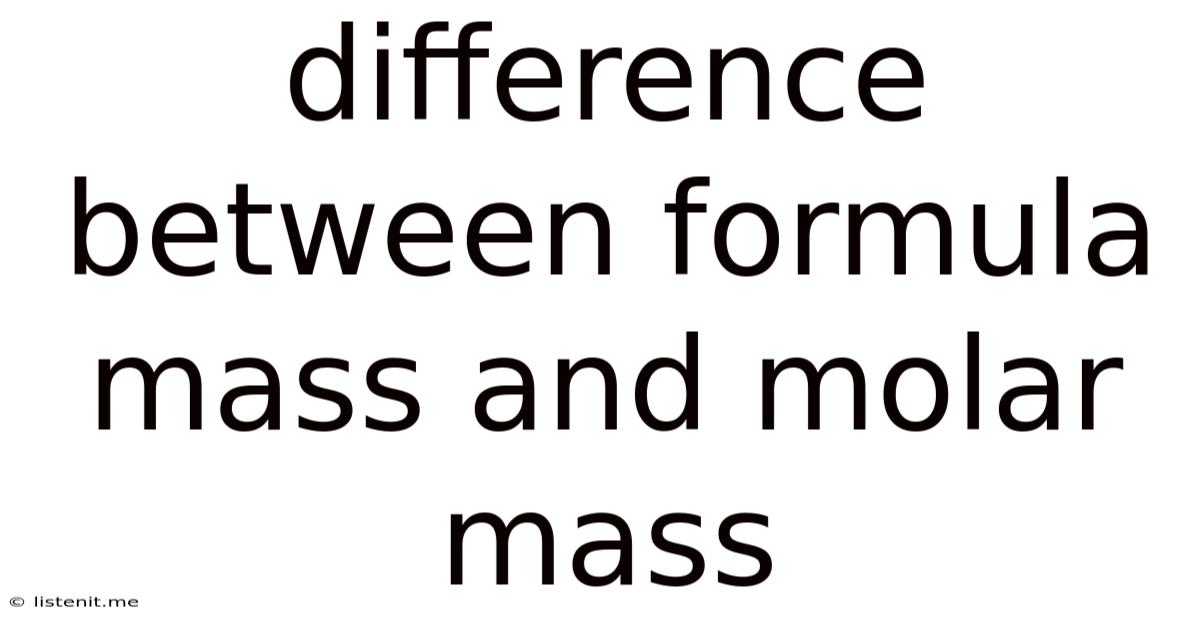
Table of Contents
Delving Deep: Formula Mass vs. Molar Mass – Understanding the Subtle Differences
Understanding the fundamental concepts in chemistry is crucial for anyone pursuing the subject, whether at a high school, undergraduate, or graduate level. Often, terms seem interchangeable, leading to confusion. This article aims to clarify the distinctions between two seemingly similar concepts: formula mass and molar mass. While closely related, they represent different aspects of the mass of a substance, and grasping this difference is key to accurate chemical calculations.
What is Formula Mass?
Formula mass, also known as formula weight, refers to the sum of the atomic masses of all atoms present in a chemical formula. This concept is primarily applied to ionic compounds and other non-molecular substances where discrete molecules don't exist in the typical sense. Instead, we consider the simplest whole-number ratio of ions in the crystal lattice structure.
Calculating Formula Mass: A Step-by-Step Guide
Calculating the formula mass involves a straightforward process:
-
Identify the chemical formula: For example, let's consider sodium chloride (NaCl).
-
Determine the atomic mass of each element: Consult a periodic table to find the atomic mass of sodium (Na) and chlorine (Cl). The atomic mass is usually given in atomic mass units (amu). Keep in mind that these are average atomic masses, accounting for the natural abundance of isotopes.
-
Multiply the atomic mass by the number of atoms: In NaCl, there's one sodium atom and one chlorine atom.
-
Sum the atomic masses: Add the atomic masses of all the atoms in the formula.
Example:
Let's calculate the formula mass of sodium chloride (NaCl):
- Atomic mass of Na ≈ 22.99 amu
- Atomic mass of Cl ≈ 35.45 amu
Formula mass of NaCl = (1 × 22.99 amu) + (1 × 35.45 amu) = 58.44 amu
Application of Formula Mass
Formula mass finds its application primarily in:
- Stoichiometric calculations: While molar mass is often preferred, formula mass can be used in calculations involving reactions of ionic compounds, though it's less common.
- Understanding the relative mass of ionic compounds: It provides a comparative measure of the mass of different ionic compounds.
- Basic chemical calculations: It helps in simpler calculations where the precise number of moles isn't the central focus.
What is Molar Mass?
Molar mass is defined as the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of entities (atoms, molecules, ions, etc.). The molar mass is expressed in grams per mole (g/mol).
Calculating Molar Mass: A Practical Approach
Calculating molar mass is conceptually similar to calculating formula mass, but with a crucial difference: the units. The value itself is numerically identical to the formula mass but expressed in grams per mole (g/mol) instead of atomic mass units (amu).
Example:
For water (H₂O):
- Atomic mass of H ≈ 1.01 amu
- Atomic mass of O ≈ 16.00 amu
Molar mass of H₂O = (2 × 1.01 g/mol) + (1 × 16.00 g/mol) = 18.02 g/mol
This means that one mole of water molecules weighs approximately 18.02 grams.
Application of Molar Mass: The Cornerstone of Chemical Calculations
Molar mass is a fundamental concept that underpins many crucial calculations in chemistry:
- Stoichiometry: It's essential for determining the amounts of reactants and products in chemical reactions.
- Concentration calculations: It's crucial for determining the molarity, molality, and other concentration units of solutions.
- Gas laws: Molar mass plays a vital role in understanding the behavior of gases, particularly in calculations involving the ideal gas law.
- Solution preparation: Accurate determination of molar mass ensures precise preparation of solutions with specific concentrations.
Key Differences Summarized: Formula Mass vs. Molar Mass
While numerically similar for many substances, formula mass and molar mass differ in their definition, units, and primary applications:
| Feature | Formula Mass | Molar Mass |
|---|---|---|
| Definition | Sum of atomic masses in a chemical formula | Mass of one mole of a substance |
| Units | Atomic mass units (amu) | Grams per mole (g/mol) |
| Application | Primarily for ionic compounds; simpler calculations | Essential for stoichiometry, concentration calculations, gas laws |
| Scope | Restricted to the relative mass of a formula unit | Extends to encompass a macroscopic amount of a substance |
Beyond the Basics: Addressing Common Misconceptions
Several misconceptions often surround formula mass and molar mass. Let's address some of the most common:
- Interchangeability: While numerically the same for many compounds, they are not interchangeable. Using amu instead of g/mol in stoichiometric calculations would yield incorrect results.
- Applicability to all substances: Formula mass is less applicable to covalent molecules where the concept of a discrete molecule is more relevant than the simplest ratio of atoms. Molar mass is universal, regardless of the substance's nature.
- Units are merely different: The difference is more fundamental than just the units. Molar mass involves Avogadro's number, linking the microscopic world of atoms and molecules to the macroscopic world of grams and moles.
Practical Examples: Illustrating the Concepts
Let's work through a few examples to solidify our understanding:
Example 1: Calculating the molar mass of glucose (C₆H₁₂O₆)
-
Identify the elements and their atomic masses: C (12.01 g/mol), H (1.01 g/mol), O (16.00 g/mol).
-
Multiply atomic masses by the number of atoms: (6 × 12.01 g/mol) + (12 × 1.01 g/mol) + (6 × 16.00 g/mol)
-
Sum the values: 180.18 g/mol. This is the molar mass of glucose.
Example 2: Determining the number of moles in 10 grams of sodium chloride (NaCl)
-
Calculate the molar mass of NaCl: (22.99 g/mol) + (35.45 g/mol) = 58.44 g/mol
-
Use the formula: moles = mass (grams) / molar mass (g/mol)
-
Solve: moles = 10 g / 58.44 g/mol ≈ 0.171 moles
Conclusion: Mastering the Nuances of Mass in Chemistry
The difference between formula mass and molar mass, while subtle, is crucial for accurate chemical calculations. Formula mass provides a relative measure of mass for a formula unit, primarily useful for ionic compounds. Molar mass, on the other hand, is the mass of one mole of a substance and serves as a cornerstone for various chemical calculations, bridging the microscopic and macroscopic worlds. Understanding these distinctions is essential for anyone seeking a solid foundation in chemistry. By mastering these concepts, you’ll significantly improve your ability to solve chemical problems and interpret chemical data. Remember to always pay attention to units and consider the context of the problem to choose the appropriate mass calculation method.
Latest Posts
Latest Posts
-
Is 2 1 3 Equal To 2 3
May 10, 2025
-
How To Find Slope From Standard Form
May 10, 2025
-
2 Over 9 As A Decimal
May 10, 2025
-
Enzymes Belong To Which Class Of Macromolecules
May 10, 2025
-
5 Protons 5 Neutrons 5 Electrons
May 10, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Formula Mass And Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.