Density Of Water At 4 Degrees Celsius
listenit
Mar 15, 2025 · 5 min read
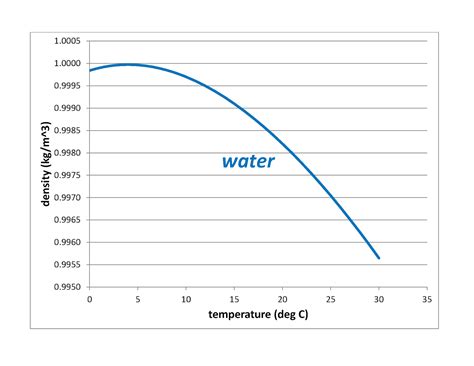
Table of Contents
The Unique Density of Water at 4 Degrees Celsius: A Deep Dive
Water, the elixir of life, is a substance so ubiquitous that we often take its remarkable properties for granted. One of the most fascinating and crucial of these properties is its density, which exhibits an unusual behavior around 4 degrees Celsius (39.2 degrees Fahrenheit). Understanding this anomaly is key to comprehending a multitude of natural phenomena, from the survival of aquatic life to the very formation of our planet's climate systems.
The Density Anomaly: Why is Water Densest at 4°C?
Unlike most substances, which continuously become denser as they cool down, water reaches its maximum density at 4°C. Below this temperature, it starts to expand, becoming less dense. This seemingly simple observation has profound implications. The reason behind this unique behavior lies in the hydrogen bonds that hold water molecules together.
The Role of Hydrogen Bonds
Water molecules (H₂O) are polar, meaning they have a slightly positive end (the hydrogen atoms) and a slightly negative end (the oxygen atom). This polarity allows them to form hydrogen bonds with neighboring molecules. At higher temperatures, these bonds are constantly breaking and reforming due to the increased kinetic energy of the molecules. As the temperature decreases, the molecules slow down, and the hydrogen bonds become more organized.
The Crystal Structure of Ice
Below 4°C, the hydrogen bonding takes on a highly ordered, crystalline structure. This structure is less dense than the more randomly organized structure found in liquid water at temperatures above 4°C. Think of it like arranging oranges in a box: you can fit more oranges in if you randomly stack them than if you arrange them in a perfectly ordered lattice. This ordered lattice in ice causes the water molecules to spread out, resulting in a lower density than liquid water at 4°C.
The Significance of the Density Maximum
This density maximum at 4°C is crucial for life on Earth. If ice were denser than water, it would sink to the bottom of lakes and oceans during winter. This would lead to the complete freezing of water bodies, making aquatic life impossible. The fact that ice floats acts as an insulating layer, preventing the underlying water from freezing solid and protecting aquatic ecosystems. This unique property plays a critical role in maintaining the delicate balance of life in aquatic environments.
Implications of Water's Density Anomaly
The anomalous density of water has far-reaching consequences across various scientific fields, impacting everything from climate regulation to geological processes.
Impact on Aquatic Ecosystems
As previously mentioned, the lower density of ice compared to water is crucial for aquatic life. The floating ice layer acts as a thermal barrier, preventing the complete freezing of water bodies. This allows aquatic organisms to survive even during harsh winters. The density variation with temperature also drives water circulation patterns in lakes and oceans, influencing nutrient distribution and oxygen levels, thereby supporting a vast array of marine and freshwater life.
Influence on Climate and Weather Patterns
Water's density anomaly plays a significant role in regulating Earth's climate. The density differences drive ocean currents, which transport heat around the globe. These currents, such as the Gulf Stream, moderate temperatures in coastal regions and influence weather patterns across continents. The thermal properties of water also moderate temperature fluctuations, making coastal climates milder than inland climates. Changes in water temperature and density can significantly impact these currents and, consequently, global weather patterns.
Geological Processes and Erosion
The density of water influences geological processes such as erosion and weathering. The freezing and thawing of water in rock crevices, driven by the density changes, causes physical weathering, breaking down rocks and shaping landscapes. The density of water also plays a role in sedimentation processes in rivers and oceans, affecting the formation of geological formations.
Applications in Various Fields
Understanding the density properties of water is essential in many fields. In oceanography, precise measurements of water density are vital for understanding ocean currents and circulation patterns. In meteorology, water density is crucial for modeling atmospheric processes and predicting weather. In engineering, understanding water's behavior at different temperatures is essential for designing and maintaining water infrastructure.
Measuring the Density of Water at 4°C
Precisely determining the density of water at 4°C requires careful experimental techniques. While the exact value is subject to slight variations depending on factors such as pressure and isotopic composition, it's generally accepted to be around 999.97 kg/m³.
Experimental Methods
Determining the density of water involves precise measurements of its mass and volume. Common methods include:
- Pycnometry: This classic method involves measuring the mass of a known volume of water using a precisely calibrated pycnometer (a specialized density bottle).
- Hydrometry: Hydrometers measure the density of liquids based on their buoyancy. However, achieving high precision requires careful calibration and temperature control.
- Other Techniques: More advanced techniques like oscillating U-tube densitometry provide highly precise density measurements, but require specialized equipment.
Factors Affecting Density
Several factors can influence the precise density measurement of water:
- Temperature: Even minor temperature fluctuations significantly impact density, necessitating very precise temperature control.
- Pressure: Increased pressure leads to slightly higher density.
- Isotopic Composition: The presence of heavier isotopes of hydrogen and oxygen (deuterium and oxygen-18) slightly alters the density.
- Dissolved Substances: Dissolved salts and other substances affect the density of water.
Further Research and Exploration
The unique density properties of water continue to fascinate researchers. Ongoing research explores the detailed microscopic behavior of water molecules near 4°C, seeking a more complete understanding of the hydrogen bonding networks and their influence on density. This research has implications for numerous fields, including materials science, nanotechnology, and biology. The study of water's anomalous properties also holds potential for developing new technologies based on its unique behavior.
Conclusion
The density of water at 4°C is far more than a mere scientific curiosity. This seemingly simple property is a fundamental aspect of our planet’s physical and biological systems. Its influence on climate, weather patterns, aquatic ecosystems, and geological processes is undeniable. Further research promises to deepen our understanding of this remarkable substance and its profound influence on our world. Understanding this density anomaly is not only crucial for scientific progress but also vital for addressing challenges related to climate change and environmental conservation. The more we learn about water, the better we can understand and protect our planet.
Latest Posts
Latest Posts
-
Do Plant Cells Have A Mitochondria
Mar 15, 2025
-
What Percent Of 40 Is 80
Mar 15, 2025
-
70 Of What Number Is 35
Mar 15, 2025
-
Words That Start With Same Letter
Mar 15, 2025
-
60 Miles Per Hour Is How Many Feet Per Second
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Density Of Water At 4 Degrees Celsius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
