Cu Agno3 Cu No3 2 Ag
listenit
May 11, 2025 · 6 min read
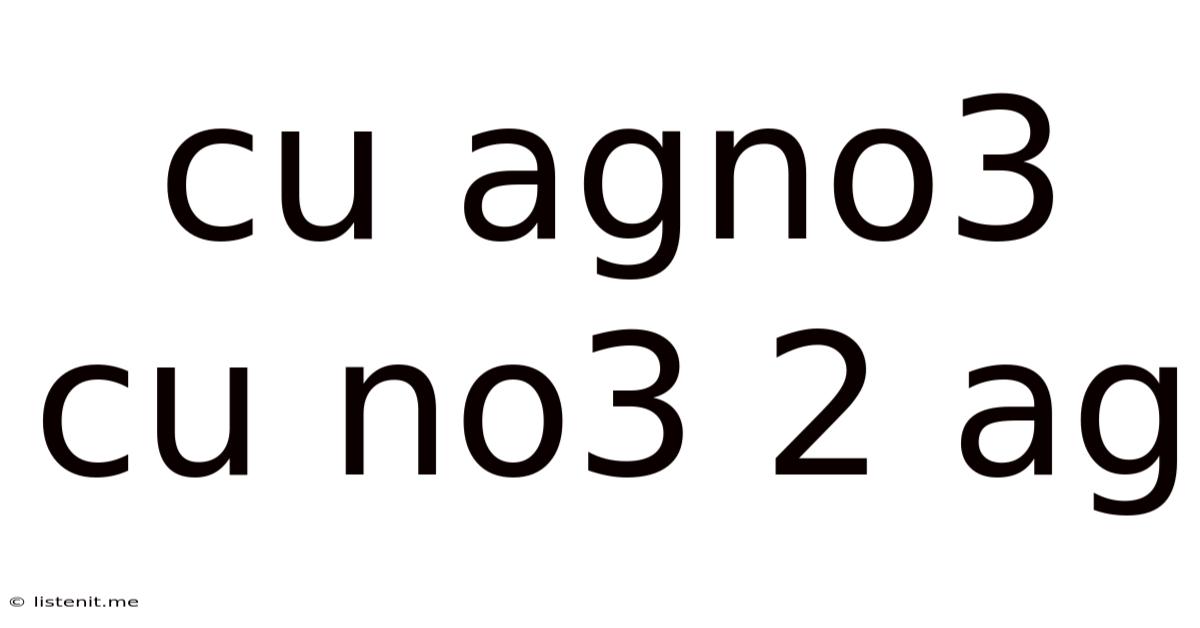
Table of Contents
Understanding the Reaction: Cu + AgNO₃ → Cu(NO₃)₂ + Ag
The reaction between copper (Cu) and silver nitrate (AgNO₃) is a classic example of a single displacement or substitution reaction, a fundamental concept in chemistry. This reaction demonstrates the relative reactivity of metals and is frequently used in educational settings to illustrate redox processes. Understanding this reaction requires a grasp of several key chemical concepts, including oxidation states, redox reactions, and stoichiometry. This article will delve into the specifics of the reaction, exploring its mechanism, applications, and relevant safety considerations.
The Reaction Mechanism: A Closer Look
The reaction between copper and silver nitrate is a redox reaction, meaning that it involves both reduction and oxidation processes. Copper, a more reactive metal than silver, displaces silver from its nitrate salt. This displacement occurs due to copper's higher tendency to lose electrons (oxidation) compared to silver.
Oxidation of Copper: Copper atoms lose two electrons to form copper(II) ions (Cu²⁺). This is represented by the half-reaction:
Cu(s) → Cu²⁺(aq) + 2e⁻
Reduction of Silver Ions: Silver ions (Ag⁺) in the silver nitrate solution gain electrons to become neutral silver atoms (Ag). This is the reduction half-reaction:
Ag⁺(aq) + e⁻ → Ag(s)
Overall Reaction: To obtain the overall balanced equation, we need to multiply the reduction half-reaction by two to balance the electrons transferred:
2Ag⁺(aq) + 2e⁻ → 2Ag(s)
Now, we can add the oxidation and reduction half-reactions together, cancelling out the electrons:
Cu(s) + 2Ag⁺(aq) → Cu²⁺(aq) + 2Ag(s)
This balanced equation shows that one atom of copper reacts with two silver ions to produce one copper(II) ion and two silver atoms. The copper(II) ions remain in solution as copper(II) nitrate, Cu(NO₃)₂, while the silver atoms precipitate out of the solution as a solid, often appearing as a silvery-grey coating on the copper.
Observable Changes During the Reaction
Visually observing this reaction is quite striking. Several noticeable changes occur as the reaction progresses:
-
Formation of Silver Coating: The most prominent change is the gradual appearance of a silvery-grey coating on the surface of the copper strip or wire. This coating consists of the solid silver metal that has been displaced from the silver nitrate solution. The coating thickness will increase over time, depending on the concentration of the silver nitrate solution and the surface area of the copper.
-
Color Change of the Solution: The initially clear, colorless silver nitrate solution will gradually change color. As the copper dissolves and forms copper(II) nitrate, the solution takes on a pale blue-green hue, characteristic of copper(II) ions in aqueous solution. The intensity of this blue-green color will increase as the reaction progresses, reflecting the increasing concentration of copper(II) ions.
-
Dissolution of Copper: The copper metal itself will gradually dissolve as it reacts with the silver nitrate. The copper strip or wire will become thinner and potentially even perforate if the reaction proceeds for a prolonged period.
Factors Affecting the Reaction Rate
Several factors influence the rate at which this reaction proceeds:
-
Concentration of Silver Nitrate: A higher concentration of silver nitrate will lead to a faster reaction rate. This is because a greater number of silver ions are available to react with the copper.
-
Surface Area of Copper: Increasing the surface area of the copper (e.g., by using copper powder instead of a solid piece) will accelerate the reaction. A larger surface area provides more sites for the reaction to occur.
-
Temperature: Raising the temperature will generally increase the reaction rate. Higher temperatures provide the reacting particles with more kinetic energy, leading to more frequent and energetic collisions.
-
Presence of Impurities: Impurities on the surface of the copper could potentially inhibit the reaction. A clean copper surface will react more readily.
Applications of the Cu + AgNO₃ Reaction
While primarily used as a demonstration in educational settings, the principles behind this reaction have practical applications:
-
Electroplating: This reaction highlights the fundamental principles behind electroplating, a process where a thin layer of metal is deposited onto a conductive surface. The displacement of silver by copper is analogous to the controlled deposition of metal ions in electroplating.
-
Extraction of Metals: The reactivity series of metals, exemplified by this reaction, is crucial in the extraction of metals from their ores. More reactive metals can be used to displace less reactive metals from their compounds, a principle used in various metallurgical processes.
-
Chemical Analysis: The reaction can be used in qualitative chemical analysis to identify the presence of silver ions in a solution. The formation of silver precipitate serves as a visual indicator of the presence of silver.
Safety Precautions
When conducting this reaction, it is crucial to observe proper safety precautions:
-
Wear Safety Goggles: Always wear safety goggles to protect your eyes from splashes of chemicals.
-
Gloves: Wear chemical-resistant gloves to prevent skin contact with the chemicals.
-
Ventilation: Perform the reaction in a well-ventilated area to avoid inhaling any fumes.
-
Disposal: Dispose of the chemical waste properly according to your local regulations. Never pour chemicals down the drain.
-
Avoid Ingestion: Avoid ingesting any of the chemicals involved in the reaction.
Expanding on the Stoichiometry
The balanced equation: Cu(s) + 2AgNO₃(aq) → Cu(NO₃)₂(aq) + 2Ag(s) indicates a specific molar ratio between the reactants and products. This is crucial for understanding the quantitative aspects of the reaction.
For instance, if we react 1 mole of copper with an excess of silver nitrate, we expect to obtain 2 moles of silver and 1 mole of copper(II) nitrate. This stoichiometric relationship allows us to predict the amount of products formed based on the amount of reactants used. Calculations involving molar mass and limiting reactants are commonly performed to fully understand the quantitative aspects of this reaction.
Example Calculation:
Let's say we react 6.35 grams of copper with an excess of silver nitrate. To determine the theoretical yield of silver, we would follow these steps:
-
Convert grams of copper to moles: The molar mass of copper is 63.5 g/mol. Therefore, 6.35 g Cu / 63.5 g/mol = 0.1 moles of Cu.
-
Use the stoichiometric ratio: According to the balanced equation, 1 mole of Cu produces 2 moles of Ag. Therefore, 0.1 moles Cu × (2 moles Ag / 1 mole Cu) = 0.2 moles of Ag.
-
Convert moles of silver to grams: The molar mass of silver is 107.9 g/mol. Therefore, 0.2 moles Ag × 107.9 g/mol = 21.58 grams of Ag.
This calculation demonstrates that reacting 6.35 grams of copper with excess silver nitrate would theoretically yield 21.58 grams of silver. In practice, the actual yield may be slightly lower due to factors like incomplete reaction or loss of product during the process.
Conclusion
The reaction between copper and silver nitrate is a fundamental example of a single displacement reaction, providing a clear demonstration of redox processes and the reactivity series of metals. By understanding the reaction mechanism, observable changes, influencing factors, and safety precautions, we can fully appreciate this simple yet insightful chemical process. Its applications extend beyond the classroom, illustrating essential principles in various chemical and metallurgical processes. The stoichiometric calculations further emphasize the quantitative aspects of the reaction, allowing for precise predictions of product yield based on reactant quantities. This reaction serves as a valuable tool for both learning and practical applications in chemistry.
Latest Posts
Latest Posts
-
Why Do Electric Field Lines Never Cross
May 13, 2025
-
1 10 As A Percent And Decimal
May 13, 2025
-
Can All Minerals Be A Gemstone
May 13, 2025
-
Multicellular Heterotrophs Without A Cell Wall
May 13, 2025
-
What Are The Gcf Of 48
May 13, 2025
Related Post
Thank you for visiting our website which covers about Cu Agno3 Cu No3 2 Ag . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.