Correct Bohr Model For A Neutral Nitrogen Atom
listenit
Mar 16, 2025 · 6 min read
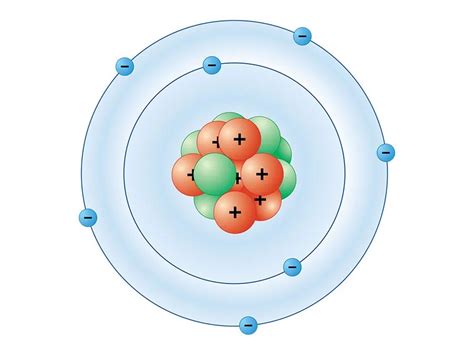
Table of Contents
The Correct Bohr Model for a Neutral Nitrogen Atom: A Deep Dive
The Bohr model, while a simplified representation of atomic structure, provides a valuable foundation for understanding electron arrangement and fundamental atomic properties. While it has limitations, particularly with more complex atoms, it's a crucial stepping stone to grasping more sophisticated quantum mechanical models. This article will meticulously detail the correct Bohr model for a neutral nitrogen atom, explaining its construction, limitations, and its significance in understanding basic atomic behavior.
Understanding the Bohr Model's Fundamentals
Before delving into nitrogen, let's refresh the core principles of the Bohr model:
- Nucleus: At the atom's center is a dense nucleus containing protons (positively charged) and neutrons (neutral).
- Electron Orbits: Electrons orbit the nucleus in specific, quantized energy levels or shells. These shells are denoted by the principal quantum number, n, where n = 1, 2, 3, and so on, representing increasing distance from the nucleus and energy level.
- Energy Levels: Electrons can only exist in these specific energy levels; they cannot reside between them. An electron can transition between energy levels by absorbing or emitting energy in the form of a photon.
- Electron Capacity: Each shell has a maximum capacity of electrons. The first shell (n=1) can hold a maximum of 2 electrons, the second shell (n=2) can hold a maximum of 8 electrons, and so on (following the 2n² rule).
Constructing the Bohr Model for a Neutral Nitrogen Atom
Nitrogen (N) has an atomic number of 7, meaning it possesses 7 protons in its nucleus. To be neutral, it must also have 7 electrons orbiting the nucleus. Let's build the Bohr model step-by-step:
-
The Nucleus: Draw a central circle representing the nucleus, containing 7 protons. While neutrons are present in a nitrogen atom's nucleus (typically 7), they aren't explicitly shown in a basic Bohr model as they don't participate in chemical bonding.
-
The First Shell (n=1): The first shell can hold a maximum of 2 electrons. Fill this shell with 2 electrons. Represent these electrons as small dots or circles orbiting the nucleus within the first shell.
-
The Second Shell (n=2): After filling the first shell, we have 5 electrons remaining (7 total electrons – 2 in the first shell = 5). The second shell can hold up to 8 electrons. Place the remaining 5 electrons into the second shell. Again, represent these electrons as small dots or circles orbiting the nucleus within the second shell.
The Completed Bohr Model: The completed model should show a nucleus with 7 protons, a first shell with 2 electrons, and a second shell with 5 electrons. This visually represents the electronic configuration of nitrogen as 1s²2s²2p³. Note that the Bohr model doesn’t differentiate between s and p orbitals (subshells); it simply shows electrons in the shell.
Visual Representation & Limitations
While a textual description is helpful, a visual representation is crucial for understanding the Bohr model of nitrogen. Imagine concentric circles representing the electron shells around a central nucleus. The inner circle (n=1) holds two electrons, and the outer circle (n=2) holds five. This visual clearly illustrates the electron distribution in the nitrogen atom according to the Bohr model.
However, the Bohr model, while conceptually simple, has several key limitations:
- Electron Orbitals: The model depicts electrons orbiting the nucleus in fixed circular paths, which is inaccurate. Quantum mechanics reveals electrons exist in orbitals, regions of space with a high probability of finding an electron. These orbitals are not simple circles but have more complex shapes (s, p, d, f).
- Electron Spin: The Bohr model doesn't account for electron spin, a fundamental quantum property affecting electron interactions and configurations.
- Multi-electron Atoms: The model struggles to accurately predict the behavior of multi-electron atoms because it doesn't account for electron-electron repulsions. These repulsions significantly influence electron energies and distributions.
- Spectral Lines: While the Bohr model explains certain aspects of atomic spectra (emission and absorption of light), it fails to accurately predict the fine structure and intensity of spectral lines observed in more complex atoms.
Beyond the Bohr Model: Quantum Mechanics and Nitrogen
While the Bohr model provides a basic understanding of electron arrangement, a more accurate representation requires quantum mechanics. Quantum mechanics describes electrons not as particles in fixed orbits, but as wave functions that occupy orbitals within electron clouds.
For Nitrogen, the quantum mechanical description elaborates on the electronic configuration:
- 1s²: Two electrons occupy the 1s orbital, the lowest energy orbital closest to the nucleus.
- 2s²: Two electrons occupy the 2s orbital, also a spherical orbital but larger and higher in energy than the 1s orbital.
- 2p³: The remaining three electrons occupy the 2p orbitals. The 2p subshell contains three degenerate orbitals (px, py, pz) oriented along the x, y, and z axes. According to Hund's rule, each 2p orbital will be singly occupied before pairing begins.
This quantum mechanical picture is far more accurate and provides a deeper understanding of nitrogen's chemical behavior and bonding properties. The Bohr model, however, remains a useful pedagogical tool for introducing the concept of atomic structure.
Nitrogen's Chemical Behavior and the Bohr Model
The Bohr model, despite its limitations, helps us understand some aspects of nitrogen's chemical behavior. The presence of three unpaired electrons in the outer shell (second shell) indicates nitrogen's tendency to form three covalent bonds. This is because these unpaired electrons can be shared with other atoms to achieve a stable octet configuration. This explains nitrogen's presence in many important molecules like ammonia (NH₃) and various organic compounds.
The strong tendency to form three bonds is directly related to the incompleteness of the outer shell in the Bohr model, visually illustrating the atom's drive towards stability.
Applications and Significance
Understanding the Bohr model, even with its shortcomings, remains crucial for several reasons:
- Educational Foundation: It serves as a fundamental introduction to atomic structure, making the transition to more complex quantum mechanical models smoother.
- Chemical Bonding: It helps visualize electron sharing and the formation of covalent bonds, although a quantum mechanical approach is necessary for more precise predictions.
- Predicting Valency: It allows us to predict the valency of elements, a basic concept in chemical bonding and reactivity. Nitrogen's valency of 3 is readily understood through the Bohr model.
- Historical Context: Understanding the Bohr model provides crucial historical context, showing the evolution of our understanding of atomic structure.
Conclusion
The Bohr model for a neutral nitrogen atom, while a simplification, provides a valuable initial understanding of atomic structure. It allows for a clear visualization of electron distribution and the basis for chemical bonding. While quantum mechanics provides a far more accurate and comprehensive description, the Bohr model remains an essential stepping stone for students and a powerful tool for basic comprehension of atomic structure and chemical behavior. Remembering its limitations is crucial for a complete understanding of atomic theory.
Latest Posts
Latest Posts
-
480 Cm Equals How Many M
Mar 17, 2025
-
What Is 8 In Fraction Form
Mar 17, 2025
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Correct Bohr Model For A Neutral Nitrogen Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
