Cobalt Ii Hydrogen Carbonate Chemical Formula
listenit
Apr 07, 2025 · 5 min read
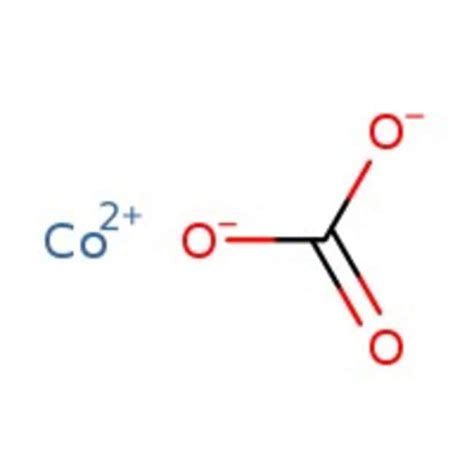
Table of Contents
Cobalt(II) Hydrogen Carbonate: A Deep Dive into its Chemical Formula, Properties, and Applications
Cobalt(II) hydrogen carbonate, a fascinating inorganic compound, holds a significant place in various chemical and industrial applications. Understanding its chemical formula, properties, and uses requires a thorough investigation. This article aims to provide a comprehensive overview, delving into the intricacies of this intriguing compound.
Understanding the Chemical Formula: Co(HCO₃)₂
The chemical formula for cobalt(II) hydrogen carbonate is Co(HCO₃)₂. Let's break this down:
-
Co: Represents the cobalt(II) ion. The Roman numeral II indicates that cobalt has a +2 oxidation state. This is crucial because cobalt can exist in different oxidation states, impacting its chemical behavior.
-
HCO₃: Represents the hydrogen carbonate ion, also known as the bicarbonate ion. This polyatomic ion carries a -1 charge.
The parentheses around HCO₃ indicate that the bicarbonate ion is a single entity, and there are two of them to balance the +2 charge of the cobalt(II) ion. The overall charge of the compound is neutral.
Is it stable? The Challenge of Isolation
While the formula Co(HCO₃)₂ is readily written, the reality of isolating and handling pure cobalt(II) hydrogen carbonate is significantly more challenging. This is due to its inherent instability. Unlike many other metal hydrogen carbonates, Co(HCO₃)₂ readily decomposes. This decomposition often results in the formation of cobalt(II) carbonate (CoCO₃) and the release of carbon dioxide (CO₂):
Co(HCO₃)₂ → CoCO₃ + CO₂ + H₂O
This inherent instability significantly impacts its applications and necessitates careful handling and storage conditions. The decomposition reaction is influenced by factors such as temperature, pressure, and the presence of water. Therefore, finding ways to stabilize the compound remains an area of ongoing research.
Physical and Chemical Properties
While isolating pure Co(HCO₃)₂ is difficult, understanding its predicted properties based on its chemical formula and the known properties of similar compounds is crucial.
Predicted Properties (Based on Related Compounds):
-
Appearance: It's likely to be a pink or reddish-colored solid, similar to other cobalt(II) salts. The exact shade might vary depending on the degree of hydration.
-
Solubility: It is expected to be sparingly soluble in water, similar to other transition metal hydrogen carbonates.
-
Thermal Stability: As mentioned, its low thermal stability leads to decomposition at relatively low temperatures.
-
Reactivity: It's anticipated to react with acids, bases, and oxidizing agents, exhibiting typical reactions of transition metal compounds and hydrogen carbonates.
-
Magnetic Properties: Cobalt(II) ions typically exhibit paramagnetic properties, meaning they are attracted to magnetic fields. This property is likely to be present in Co(HCO₃)₂.
Potential Applications (Considering its Instability):
Despite its instability, the potential applications of cobalt(II) hydrogen carbonate, or compounds derived from its decomposition, are worth considering. However, it's important to remember that these applications are mostly theoretical or inferred from the properties of similar, more stable compounds.
1. Precursor for Other Cobalt Compounds:
The decomposition of Co(HCO₃)₂ can be a controlled pathway to synthesize other valuable cobalt compounds. The resulting cobalt(II) carbonate, for example, could serve as a precursor for various catalytic materials, pigments, and other chemical compounds. The controlled decomposition allows for the creation of highly pure cobalt-containing materials.
2. Catalytic Applications:
Cobalt compounds are known for their catalytic activity in various chemical reactions. Although direct use of Co(HCO₃)₂ might be impractical due to its instability, its decomposition products could exhibit catalytic properties. Specific catalytic applications would depend on the precise nature of the reaction and the presence of other components in the catalytic system.
3. Pigment Production (Indirectly):
The pink/reddish hue associated with cobalt(II) compounds could potentially be exploited in pigment production, though indirectly. The decomposition products could serve as precursors for pigments after further processing and refinement. The resulting pigments could be utilized in paints, ceramics, and other applications.
4. Electrochemical Applications (Speculative):
Given the redox properties of cobalt, exploring its potential in electrochemical applications is an area of ongoing research. While Co(HCO₃)₂ itself may not be directly suitable, derived materials after careful processing could find niches in batteries or other electrochemical systems.
Research and Future Directions:
Overcoming the instability of Co(HCO₃)₂ is a key challenge for researchers. Several avenues of research are being pursued:
-
Stabilization Strategies: Investigating methods to stabilize Co(HCO₃)₂ through complexation with other ligands or by controlling reaction conditions is crucial. This could involve incorporating it into a matrix or using specific solvents.
-
Controlled Decomposition: Precise control over the decomposition pathway could allow for the creation of finely tuned cobalt-containing materials with specific properties. This requires a deep understanding of the reaction kinetics and thermodynamics.
-
Exploring Analogous Compounds: Studying the properties of analogous metal hydrogen carbonates, particularly those of metals in the same group as cobalt, could offer insights into improving the stability of Co(HCO₃)₂.
Safety Considerations:
Cobalt compounds, while useful, should be handled with care. They may pose certain health risks. Always consult relevant safety data sheets (SDS) and follow appropriate safety protocols when working with cobalt compounds or their precursors. Proper ventilation and protective equipment are essential.
Conclusion:
Cobalt(II) hydrogen carbonate, despite its inherent instability, remains a fascinating chemical species. Its predicted properties and potential applications, while largely theoretical for the pure compound, highlight its significance in the broader context of cobalt chemistry. Future research focused on stabilization and controlled decomposition holds the key to unlocking its full potential in various applications, contributing to advances in catalysis, materials science, and other related fields. Further research into controlled synthesis and the characterization of Co(HCO₃)₂ under specific conditions will greatly enhance our understanding and pave the way for practical utilization. The journey to harnessing its potential is a testament to the ongoing development within the field of inorganic chemistry.
Latest Posts
Latest Posts
-
What Is X 1 X 1
Apr 08, 2025
-
What Is The Gcf Of 8 12
Apr 08, 2025
-
Dna Is Called The Blueprint Of Life Because
Apr 08, 2025
-
Difference Between Molar Mass And Atomic Mass
Apr 08, 2025
-
What Is The Greatest Common Factor Of 64 And 32
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Cobalt Ii Hydrogen Carbonate Chemical Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.