Chemical Reaction Between Hcl And Naoh
listenit
Apr 06, 2025 · 6 min read
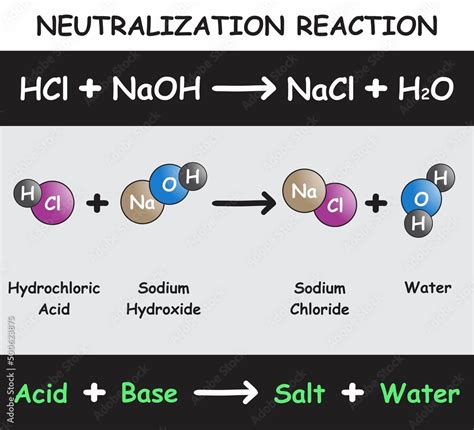
Table of Contents
The Chemical Reaction Between HCl and NaOH: A Deep Dive
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction, a fundamental concept in chemistry. Understanding this reaction provides a strong foundation for grasping broader concepts in acid-base chemistry, stoichiometry, and thermodynamics. This article will delve deep into this seemingly simple reaction, exploring its mechanism, applications, and practical implications.
Understanding the Reactants: HCl and NaOH
Before diving into the reaction itself, let's examine the properties of the individual reactants: hydrochloric acid and sodium hydroxide.
Hydrochloric Acid (HCl)
Hydrochloric acid is a strong, monoprotic acid. This means it completely dissociates in water, releasing a hydrogen ion (H⁺) and a chloride ion (Cl⁻):
HCl(aq) → H⁺(aq) + Cl⁻(aq)
Its strong acidic nature is due to the readily available proton (H⁺). HCl is a highly corrosive substance and must be handled with care. It finds widespread use in various industrial processes, including metal cleaning, leather processing, and food processing.
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as caustic soda or lye, is a strong base. It also fully dissociates in water, yielding a sodium ion (Na⁺) and a hydroxide ion (OH⁻):
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
The presence of the hydroxide ion (OH⁻) is responsible for its strong basicity. NaOH is highly corrosive and alkaline. It's extensively used in various applications, including soap making, paper production, and drain cleaning.
The Neutralization Reaction: HCl + NaOH
The reaction between HCl and NaOH is a neutralization reaction because it involves the reaction of an acid (HCl) and a base (NaOH) to form salt and water. The balanced chemical equation for this reaction is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation shows that one mole of hydrochloric acid reacts with one mole of sodium hydroxide to produce one mole of sodium chloride (NaCl) – common table salt – and one mole of water (H₂O).
The Ionic Equation
A more detailed representation of the reaction is provided by the ionic equation:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
This equation shows the complete dissociation of both the acid and the base into their constituent ions. Notice that the sodium (Na⁺) and chloride (Cl⁻) ions appear on both sides of the equation. These are spectator ions, meaning they don't directly participate in the reaction. They are present in the solution but don't undergo any chemical change.
The Net Ionic Equation
Removing the spectator ions from the ionic equation yields the net ionic equation:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This equation highlights the essence of the neutralization reaction: the combination of hydrogen ions (H⁺) and hydroxide ions (OH⁻) to form water. This is the crucial step that determines the pH change during the reaction.
Energetics of the Reaction: Exothermic Nature
The reaction between HCl and NaOH is exothermic, meaning it releases heat. When the acid and base solutions are mixed, the temperature of the resulting solution increases. This heat release is due to the formation of strong ionic bonds in the water molecule. The energy released is often significant enough to be easily measurable, making this reaction a useful illustration of thermochemistry principles in educational settings.
Enthalpy Change (ΔH)
The enthalpy change (ΔH) for this reaction is negative, indicating an exothermic process. The exact value of ΔH depends on the concentrations and quantities of reactants involved, but it is typically a substantial negative value, representing a significant release of heat.
Titration: A Practical Application
The reaction between HCl and NaOH forms the basis of acid-base titrations. Titration is a quantitative analytical technique used to determine the concentration of an unknown solution (either acid or base) using a solution of known concentration (standard solution).
In a typical titration involving HCl and NaOH, a known volume of NaOH solution is carefully added to a known volume of HCl solution using a burette. An indicator, such as phenolphthalein, is added to the HCl solution. The indicator changes color at the equivalence point, the point at which the moles of acid and base are equal, signifying complete neutralization. By measuring the volume of NaOH solution required to reach the equivalence point, the concentration of the HCl solution can be calculated using stoichiometric principles.
Applications of the HCl and NaOH Reaction
The neutralization reaction between HCl and NaOH, besides its importance in titration, has various other applications:
- Wastewater treatment: Industrial wastewater often contains acidic or basic components. Neutralization using HCl or NaOH helps adjust the pH to environmentally acceptable levels before discharge.
- Chemical synthesis: This reaction can be a part of larger synthetic schemes in organic chemistry where specific pH conditions are needed for certain steps.
- Food processing: In some food processing applications, pH control is crucial, and this reaction might be used to adjust the acidity or alkalinity of food products.
- Laboratory procedures: Many laboratory experiments require precise pH control, and neutralization reactions involving HCl and NaOH are frequently used to achieve this.
Safety Precautions
Both HCl and NaOH are corrosive substances and should be handled with extreme caution. Appropriate safety equipment, including safety goggles, gloves, and lab coats, must always be worn when working with these chemicals. Spills should be cleaned up immediately and appropriately. Always follow proper laboratory safety procedures.
Exploring Further: Beyond the Basics
While this reaction appears straightforward, deeper investigation can reveal further complexities. Factors such as temperature, concentration, and the presence of other ions can subtly influence the reaction rate and equilibrium. Furthermore, the study of kinetics and equilibrium constants related to this reaction provide a rich opportunity to explore the fundamental principles of chemical thermodynamics and reaction dynamics. Advanced studies might involve exploring the ionic strength's effect on the reaction rate, or the use of sophisticated instrumentation to measure the precise heat changes during the neutralization process.
Conclusion
The reaction between HCl and NaOH is a fundamental and vital chemical reaction with broad implications across various fields. Understanding its mechanism, energetics, and practical applications provides a solid foundation for comprehending more complex chemical phenomena. This seemingly simple reaction serves as a gateway to exploring the fascinating world of acid-base chemistry and its significant role in the natural world and our technological advancements. From the precise measurements of titrations to the crucial role in maintaining pH balances in various industrial processes, the HCl and NaOH reaction showcases the power and elegance of fundamental chemical principles. The exothermic nature of the reaction offers a valuable learning opportunity in thermochemistry, highlighting the energy changes associated with bond formation and breakage. Mastering the concepts associated with this reaction is a cornerstone of a strong understanding in chemistry.
Latest Posts
Latest Posts
-
A Neutral Atom Has The Same Number Of
Apr 08, 2025
-
Distance Between Earth And Mars In Light Years
Apr 08, 2025
-
What Is The Pka Of Hcl
Apr 08, 2025
-
What Is The Name For Fecl3
Apr 08, 2025
-
Lowest Common Denominator Of 10 And 15
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Chemical Reaction Between Hcl And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.