Ch4 O2 Co2 H2o Balanced Equation
listenit
Mar 24, 2025 · 5 min read
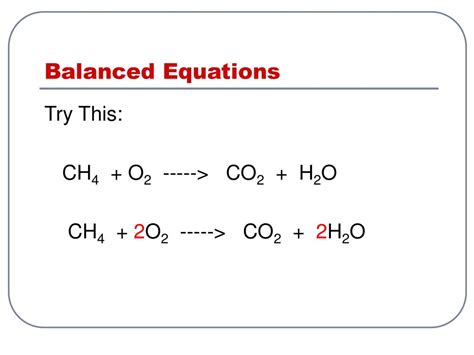
Table of Contents
The Balanced Equation: CH₄ + O₂ → CO₂ + H₂O and its Significance
The seemingly simple chemical equation, CH₄ + O₂ → CO₂ + H₂O, represents a reaction of monumental importance: the combustion of methane. Understanding this equation, balancing it correctly, and appreciating its implications in various fields is crucial for anyone studying chemistry, environmental science, or energy production. This comprehensive guide delves into the balanced equation, explores its stoichiometry, discusses its applications, and examines its environmental impact.
Understanding the Reactants and Products
Before diving into the balanced equation, let's define the chemical species involved:
-
CH₄ (Methane): A simple hydrocarbon, the primary component of natural gas. It's a colorless, odorless gas, highly flammable and a potent greenhouse gas.
-
O₂ (Oxygen): A diatomic molecule essential for respiration and combustion. It's a colorless, odorless gas that makes up approximately 21% of Earth's atmosphere.
-
CO₂ (Carbon Dioxide): A product of complete combustion. It's a colorless, odorless gas, also a significant greenhouse gas contributing to climate change.
-
H₂O (Water): Another product of complete combustion. It exists in various states (solid, liquid, gas) depending on the temperature.
Balancing the Chemical Equation: The Law of Conservation of Mass
The unbalanced equation, CH₄ + O₂ → CO₂ + H₂O, violates the fundamental law of conservation of mass. This law states that matter cannot be created or destroyed in a chemical reaction; the total mass of reactants must equal the total mass of products. To achieve balance, we must adjust the stoichiometric coefficients – the numbers placed before each chemical formula.
The balanced equation is:
CH₄ + 2O₂ → CO₂ + 2H₂O
This equation now satisfies the law of conservation of mass. Let's verify:
- Reactants: 1 carbon atom, 4 hydrogen atoms, and 4 oxygen atoms.
- Products: 1 carbon atom, 4 hydrogen atoms, and 4 oxygen atoms.
Stoichiometry: The Quantitative Relationships
Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction. The balanced equation provides the basis for stoichiometric calculations. For instance:
-
Mole Ratio: The balanced equation shows a 1:2:1:2 mole ratio between CH₄, O₂, CO₂, and H₂O, respectively. This means that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
-
Mass Relationships: Using the molar masses of the involved species, we can calculate the mass of reactants needed or products formed in a given reaction. For example, we can determine how many grams of oxygen are required to completely combust a specific mass of methane.
-
Limiting Reactant: In a reaction with more than one reactant, one reactant will be completely consumed before the others. This is the limiting reactant, determining the maximum amount of product that can be formed. The other reactants are in excess.
-
Percent Yield: The actual yield of a reaction (the amount of product obtained) is often less than the theoretical yield (calculated from stoichiometry). Percent yield reflects the efficiency of the reaction: (Actual Yield / Theoretical Yield) x 100%.
Applications of the Methane Combustion Reaction
The combustion of methane has widespread applications across various industries:
-
Energy Production: Methane is a significant fuel source for electricity generation in power plants. Burning methane in a controlled environment produces heat, which is used to generate steam that drives turbines, thus producing electricity.
-
Heating and Cooking: Natural gas, primarily methane, is commonly used for residential and commercial heating and cooking. Its efficient combustion provides heat for warming homes and preparing food.
-
Industrial Processes: Methane combustion is employed in various industrial processes requiring high temperatures, such as in the production of certain chemicals and materials.
-
Transportation: Although less common than gasoline or diesel, compressed natural gas (CNG) vehicles utilize methane as a fuel source, offering a cleaner-burning alternative.
Environmental Impact: Greenhouse Gas Emissions
While methane combustion provides essential energy, it's crucial to acknowledge its environmental impact:
-
Greenhouse Gas Emissions: The combustion of methane produces carbon dioxide, a potent greenhouse gas. The release of CO₂ contributes to climate change and global warming. However, it's important to note that methane itself is a much more potent greenhouse gas than CO₂. Therefore, burning methane, while producing CO₂, actually reduces the overall greenhouse effect compared to simply releasing the methane into the atmosphere. This is because a molecule of methane traps far more heat than a molecule of carbon dioxide.
-
Air Pollution: Incomplete combustion of methane can produce pollutants like carbon monoxide (CO), a highly toxic gas, and particulate matter, which contribute to respiratory problems.
-
Mitigation Strategies: Efforts are underway to mitigate the environmental impact of methane combustion. These include improving combustion efficiency to reduce CO₂ emissions, capturing and storing CO₂, and exploring alternative energy sources to lessen our reliance on fossil fuels. Developing technologies to capture methane leaks from natural gas infrastructure is also critically important.
Beyond the Basics: Incomplete Combustion and Other Reactions
The equation CH₄ + 2O₂ → CO₂ + 2H₂O represents complete combustion. However, under conditions with limited oxygen supply, incomplete combustion can occur, producing carbon monoxide (CO) and/or soot (carbon, C). Examples of incomplete combustion equations include:
- 2CH₄ + 3O₂ → 2CO + 4H₂O
- CH₄ + O₂ → C + 2H₂O
These reactions highlight the importance of adequate oxygen supply for efficient and safe methane combustion.
The Role of Catalysts
Catalysts can influence the rate of the methane combustion reaction. While not directly part of the balanced equation, catalysts speed up the reaction without being consumed themselves. This can be crucial in industrial applications where rapid and efficient combustion is desired.
Conclusion: A Fundamental Reaction with Far-Reaching Consequences
The balanced equation CH₄ + 2O₂ → CO₂ + 2H₂O represents a fundamental chemical reaction with significant implications for energy production and environmental sustainability. Understanding its stoichiometry, applications, and environmental consequences is essential for informed decision-making in addressing global energy needs and mitigating climate change. Continued research and technological advancements are crucial in optimizing methane combustion for cleaner energy production while minimizing its environmental impact. The pursuit of cleaner energy technologies and improved combustion efficiency remains vital for a sustainable future. Further research into alternative fuel sources and carbon capture technologies are also essential components in mitigating the long-term environmental consequences associated with methane combustion. By carefully considering the balanced equation and its implications, we can move towards a future where energy production is both efficient and environmentally responsible.
Latest Posts
Latest Posts
-
What Is The Squar Root Of 81
Mar 26, 2025
-
14 Is 20 Of What Number
Mar 26, 2025
-
Which Level Of Taxonomy Has The Fewest Organisms
Mar 26, 2025
-
What Is The Least Common Multiple Of 9 And 5
Mar 26, 2025
-
Derivative Of Square Root Of Xy
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Ch4 O2 Co2 H2o Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
