Can Ammonia Be Decomposed By A Chemical Change
listenit
Apr 06, 2025 · 5 min read
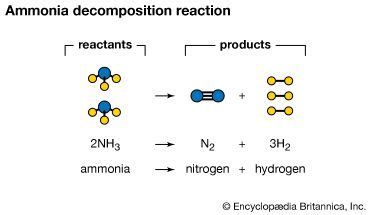
Table of Contents
Can Ammonia Be Decomposed by a Chemical Change? A Comprehensive Exploration
Ammonia (NH₃), a colorless gas with a pungent odor, is a ubiquitous compound with significant industrial applications. Understanding its chemical properties, including its decomposition, is crucial for various fields, from fertilizer production to environmental science. This article delves deep into the decomposition of ammonia, exploring the various methods, factors influencing the process, and its implications.
The Nature of Chemical Decomposition
Before exploring the decomposition of ammonia, let's establish a clear understanding of chemical decomposition itself. Chemical decomposition, also known as chemical breakdown or analysis, is a type of chemical reaction where a single compound breaks down into two or more simpler substances. This process is often driven by the addition of energy, such as heat, electricity, or light, or by the introduction of a catalyst. The fundamental characteristic of decomposition is the breaking of chemical bonds within the original compound, resulting in the formation of new products with different chemical properties.
Unlike physical changes, which only alter the form or appearance of a substance without changing its chemical composition (like melting ice), chemical decomposition fundamentally alters the chemical structure of the substance. This alteration is irreversible without further chemical reactions.
Decomposing Ammonia: Methods and Mechanisms
Ammonia, while relatively stable under normal conditions, can be decomposed through several chemical processes. The most common methods involve applying energy to break the strong nitrogen-hydrogen bonds within the molecule. Let's examine the primary methods:
1. Thermal Decomposition
Thermal decomposition, as the name suggests, uses heat to break down ammonia. This is a widely studied method, and the reaction proceeds as follows:
2NH₃(g) → N₂(g) + 3H₂(g)
This reaction is endothermic, meaning it requires an input of heat energy to proceed. The higher the temperature, the faster the rate of decomposition. However, excessively high temperatures can lead to unwanted side reactions, further complicating the process. The optimal temperature range for efficient decomposition varies based on the specific setup and desired yield. The presence of a catalyst can significantly lower the activation energy required for the reaction, making thermal decomposition more efficient at lower temperatures.
Factors influencing thermal decomposition:
- Temperature: Higher temperatures accelerate the reaction rate, leading to faster decomposition.
- Pressure: Lower pressures generally favor decomposition, as it reduces the likelihood of recombination of nitrogen and hydrogen.
- Catalyst: Certain catalysts, such as iron, nickel, and platinum, can significantly enhance the rate of thermal decomposition. The catalyst works by providing an alternative reaction pathway with a lower activation energy.
- Concentration: A higher concentration of ammonia will generally lead to a faster rate of decomposition, given the increased number of reactant molecules.
2. Electrolytic Decomposition
Electrolysis, the use of an electric current to drive a non-spontaneous chemical reaction, can also decompose ammonia. In this method, an electric current is passed through molten or dissolved ammonia, leading to its breakdown into its constituent elements. The reaction is more complex than thermal decomposition and involves the formation of intermediate ions. The process is less commonly employed compared to thermal decomposition due to its higher energy requirements and complexity.
Challenges of electrolytic decomposition:
- High energy consumption: Electrolysis requires a significant amount of electrical energy.
- Electrode selection: Choosing appropriate electrodes that are resistant to corrosion and do not interfere with the reaction is crucial.
- Complex reaction pathway: The reaction mechanism is complex and involves several intermediate steps, making it difficult to control and optimize.
3. Photochemical Decomposition
Photochemical decomposition uses light energy to break down ammonia. The process involves the absorption of photons by ammonia molecules, exciting them to a higher energy state, ultimately leading to bond breakage and decomposition. This method is often less efficient than thermal decomposition and is influenced by the intensity and wavelength of light. The presence of certain photocatalysts can significantly improve the efficiency of photochemical decomposition.
Factors influencing photochemical decomposition:
- Light intensity: Higher light intensity generally increases the rate of decomposition.
- Wavelength of light: Specific wavelengths are more effective in exciting ammonia molecules and initiating decomposition.
- Photocatalyst: Certain materials can act as photocatalysts, enhancing the rate of decomposition.
Applications and Implications of Ammonia Decomposition
The decomposition of ammonia has several important applications across various industries:
- Hydrogen production: The thermal decomposition of ammonia is a promising method for producing hydrogen, a clean energy carrier. This process can provide a sustainable source of hydrogen, reducing reliance on fossil fuels.
- Nitrogen fixation: The decomposition of ammonia can be a part of reverse nitrogen fixation processes, aiming to convert atmospheric nitrogen into usable forms.
- Environmental remediation: Ammonia decomposition plays a role in controlling ammonia emissions from industrial processes and agricultural sources.
- Chemical synthesis: The hydrogen and nitrogen produced from ammonia decomposition can be used as raw materials in various chemical syntheses.
Safety Considerations
Working with ammonia requires careful attention to safety procedures due to its hazardous nature. Ammonia is toxic and can cause irritation to the eyes, skin, and respiratory tract. In high concentrations, it can be lethal. Proper ventilation is crucial when handling ammonia, and appropriate personal protective equipment (PPE), such as gloves, goggles, and respirators, should always be used. When performing ammonia decomposition experiments, appropriate safety precautions and containment measures must be followed to prevent the release of harmful gases.
Conclusion
Ammonia decomposition through chemical change is a significant process with various implications. While thermal decomposition remains the most prevalent method, electrolytic and photochemical decomposition offer alternative approaches. The choice of method depends on various factors, including energy availability, cost considerations, and desired product purity. The hydrogen and nitrogen produced from ammonia decomposition hold immense potential for sustainable energy and chemical synthesis. However, safety precautions are paramount when handling ammonia due to its toxic nature. Future research will likely focus on developing more efficient and environmentally friendly methods for ammonia decomposition, further enhancing its role in various industrial and environmental applications. Understanding the complexities of ammonia decomposition is crucial for advancements in numerous scientific and technological fields. Further research into optimizing the reaction conditions and developing more efficient catalysts will be crucial in harnessing the full potential of this process. The quest for cleaner, sustainable energy sources and more environmentally friendly industrial practices makes the study of ammonia decomposition an increasingly relevant area of research.
Latest Posts
Latest Posts
-
What Is The Difference Between A Strong And Weak Base
Apr 07, 2025
-
How To Find Zeros Of A Cubic Function
Apr 07, 2025
-
How Many Protons Are There In Any Chlorine Atom
Apr 07, 2025
-
What Is The Lcm For 16 And 24
Apr 07, 2025
-
An Astronauts Weight On Earth Is 800 N
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Can Ammonia Be Decomposed By A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
