Calculate The Number Of Molecules In 9.00 Moles H2s .
listenit
May 09, 2025 · 5 min read
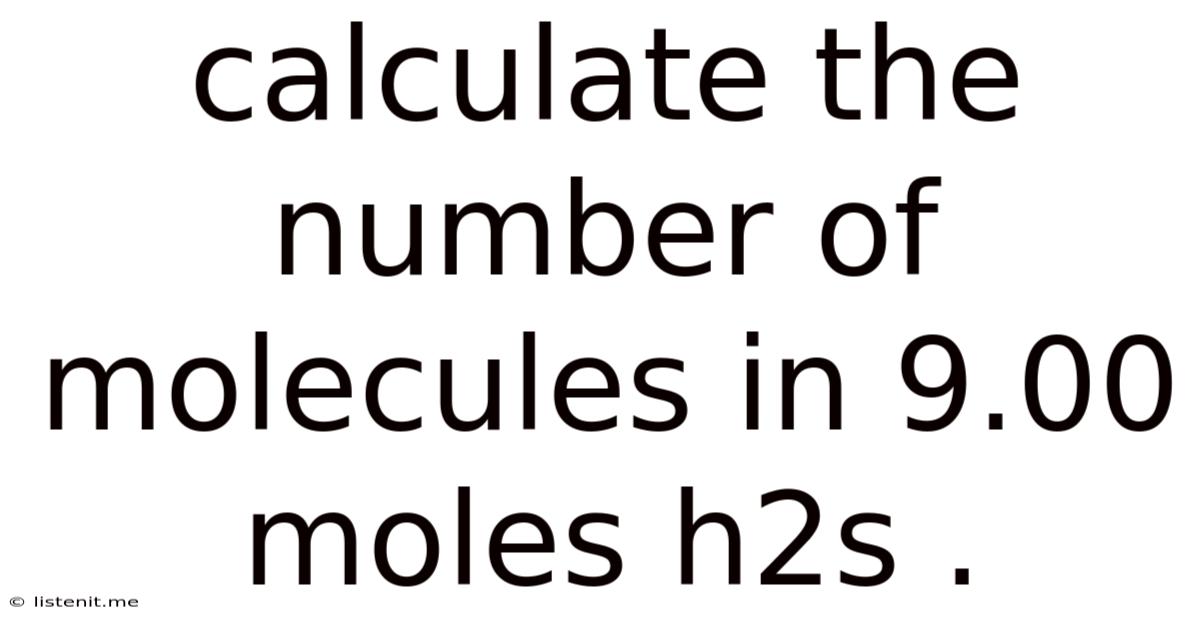
Table of Contents
Calculating the Number of Molecules in 9.00 Moles of H₂S: A Comprehensive Guide
Understanding the relationship between moles, molecules, and Avogadro's number is fundamental in chemistry. This article provides a detailed explanation of how to calculate the number of molecules in 9.00 moles of hydrogen sulfide (H₂S), covering the underlying concepts and offering practical steps for similar calculations. We'll also explore related concepts and delve into the broader implications of this type of calculation in various chemical contexts.
Understanding Moles and Avogadro's Number
Before diving into the calculation, let's clarify the key concepts:
-
Mole (mol): A mole is the International System of Units (SI) base unit for the amount of substance. It represents a specific number of particles, whether atoms, molecules, ions, or other elementary entities. This number is remarkably large!
-
Avogadro's Number (N<sub>A</sub>): This fundamental constant defines the number of entities in one mole of a substance. Its value is approximately 6.022 x 10<sup>23</sup>. This means one mole of anything contains approximately 6.022 x 10<sup>23</sup> of those things.
-
Molar Mass: The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's calculated by summing the atomic masses of all atoms in the molecule. For H₂S, we'd add the atomic mass of two hydrogen atoms and one sulfur atom.
Calculating the Number of Molecules in 9.00 Moles of H₂S
The calculation is straightforward: we'll use Avogadro's number as the conversion factor between moles and the number of molecules.
Step 1: State the given information.
We are given 9.00 moles of H₂S.
Step 2: Apply Avogadro's Number.
One mole of any substance contains 6.022 x 10<sup>23</sup> entities. Therefore, 9.00 moles of H₂S will contain:
9.00 moles H₂S × (6.022 x 10<sup>23</sup> molecules H₂S / 1 mole H₂S)
Step 3: Perform the calculation.
Multiplying 9.00 by Avogadro's number gives us:
5.4198 x 10<sup>24</sup> molecules of H₂S
Step 4: Express the answer with significant figures.
Since our initial value (9.00 moles) has three significant figures, we should round our answer to three significant figures as well. Therefore, the final answer is:
5.42 x 10<sup>24</sup> molecules of H₂S
Further Exploration: Molar Mass and Related Calculations
While the above calculation directly uses the number of moles, understanding the molar mass of H₂S allows us to perform related calculations.
Calculating the Molar Mass of H₂S:
- Atomic mass of Hydrogen (H): approximately 1.01 g/mol
- Atomic mass of Sulfur (S): approximately 32.07 g/mol
Molar mass of H₂S = (2 x 1.01 g/mol) + (1 x 32.07 g/mol) = 34.09 g/mol
This means that one mole of H₂S weighs approximately 34.09 grams.
Calculating the Mass of 9.00 Moles of H₂S:
Using the molar mass, we can calculate the mass of 9.00 moles of H₂S:
9.00 moles H₂S × 34.09 g/mol = 306.81 g
This tells us that 9.00 moles of H₂S has a mass of approximately 306.81 grams.
Applications and Importance of Mole Calculations
Understanding mole calculations is crucial in various aspects of chemistry and related fields:
1. Stoichiometry:
Stoichiometry is the quantitative study of reactants and products in chemical reactions. Knowing the number of moles of a substance is essential for determining the amounts of other reactants or products involved in a reaction. For example, if we know the number of moles of H₂S reacting with oxygen, we can determine the number of moles of sulfur dioxide and water produced.
2. Solution Chemistry:
Molarity, a common unit of concentration, is defined as moles of solute per liter of solution. Calculations involving molarity often require converting between moles and other units like grams or liters.
3. Gas Laws:
The Ideal Gas Law (PV = nRT) relates pressure, volume, temperature, and the number of moles of a gas. Being able to calculate the number of moles allows us to predict the behavior of gases under different conditions.
4. Titration:**
In titrations, we use mole calculations to determine the concentration of an unknown solution by reacting it with a solution of known concentration.
5. Pharmaceutical and Industrial Applications:**
Precise measurements of reactants in various chemical processes require the use of moles and Avogadro's number for accurate and efficient production.
Advanced Considerations and Potential Pitfalls
While the calculation for 9.00 moles of H₂S is relatively straightforward, several factors can introduce complexity:
-
Significant Figures: Always pay attention to significant figures throughout your calculations to ensure your final answer reflects the accuracy of your measurements.
-
Non-Ideal Gases: The Ideal Gas Law provides an approximation of gas behavior. At high pressures or low temperatures, gases deviate from ideal behavior, requiring more complex calculations.
-
Complex Chemical Reactions: For reactions involving multiple steps or complex stoichiometry, careful tracking of moles and balancing of chemical equations are crucial for accurate results.
-
Isotopes: The atomic masses used in molar mass calculations are weighted averages of the isotopes of each element. If you are dealing with a sample enriched in a particular isotope, you'll need to adjust the molar mass accordingly.
-
Experimental Errors: Real-world measurements always have some degree of error. Understanding and accounting for experimental error is important when interpreting results from mole-based calculations.
Conclusion
Calculating the number of molecules in 9.00 moles of H₂S, or any other substance, is a fundamental skill in chemistry. Understanding the relationship between moles, Avogadro's number, and molar mass is key to mastering stoichiometry and various other chemical calculations. By mastering these calculations, we gain a powerful tool for understanding and predicting chemical processes across a wide range of applications. This article has comprehensively covered the calculation process, explored its implications, and discussed relevant advanced considerations to ensure a thorough understanding of this crucial concept. Remember to always pay attention to significant figures and be mindful of potential sources of error.
Latest Posts
Latest Posts
-
What Are The Angle Measures Of Triangle Abc
May 10, 2025
-
How To Solve Y 2x 3
May 10, 2025
-
Average Rate Of Change Vs Slope
May 10, 2025
-
Which Subatomic Particles Have Approximately The Same Mass
May 10, 2025
-
How To Find Moles Of Acetic Acid In Vinegar
May 10, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Number Of Molecules In 9.00 Moles H2s . . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.