Calculate The Molecular Mass Of Koh
listenit
Apr 08, 2025 · 6 min read
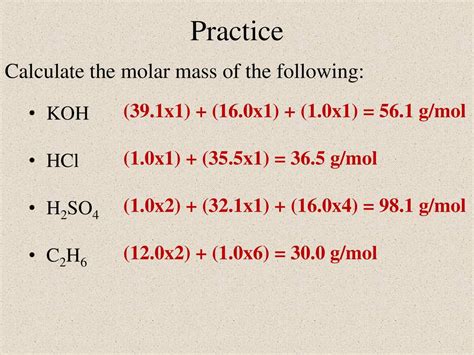
Table of Contents
Calculating the Molecular Mass of KOH: A Comprehensive Guide
Potassium hydroxide (KOH), also known as caustic potash, is a highly alkaline inorganic compound with numerous applications in various industries. Understanding its molecular mass is crucial for various chemical calculations and stoichiometric analyses. This comprehensive guide will walk you through the step-by-step process of calculating the molecular mass of KOH, explaining the underlying principles and offering helpful tips for similar calculations.
Understanding Molecular Mass
Before diving into the calculation, let's clarify the concept of molecular mass. Molecular mass, also known as molecular weight, represents the total mass of all the atoms present in a molecule. It's expressed in atomic mass units (amu) or Daltons (Da). Unlike molar mass (which we'll discuss later), molecular mass focuses on a single molecule. The crucial difference is the unit of measurement: amu for molecular mass and g/mol for molar mass.
For ionic compounds like KOH, the term "formula mass" is often used interchangeably with "molecular mass". Since KOH exists as ions in solution (K⁺ and OH⁻), "formula mass" is technically more accurate. However, for simplicity, we will continue to use "molecular mass" throughout this article.
Determining Atomic Masses
The cornerstone of calculating molecular mass lies in knowing the atomic masses of the constituent elements. These values are typically found on the periodic table of elements. Each element has an average atomic mass that reflects the abundance of its isotopes. For KOH, we need the atomic masses of:
- Potassium (K): Approximately 39.10 amu
- Oxygen (O): Approximately 16.00 amu
- Hydrogen (H): Approximately 1.01 amu
These values can vary slightly depending on the source (different periodic tables may have minor discrepancies due to rounding). For this calculation, we'll use these values. It's always best to use the most up-to-date periodic table available.
Calculating the Molecular Mass of KOH
Now, let's calculate the molecular mass of KOH. The process is straightforward:
-
Identify the number of atoms of each element: In one molecule of KOH, there's:
- 1 potassium (K) atom
- 1 oxygen (O) atom
- 1 hydrogen (H) atom
-
Multiply the number of atoms by the respective atomic mass:
- Potassium: 1 atom * 39.10 amu/atom = 39.10 amu
- Oxygen: 1 atom * 16.00 amu/atom = 16.00 amu
- Hydrogen: 1 atom * 1.01 amu/atom = 1.01 amu
-
Sum up the individual atomic masses:
- Total molecular mass = 39.10 amu + 16.00 amu + 1.01 amu = 56.11 amu
Therefore, the molecular mass of KOH is approximately 56.11 amu.
Understanding Molar Mass and its Relationship to Molecular Mass
While molecular mass focuses on a single molecule, molar mass refers to the mass of one mole (Avogadro's number, approximately 6.022 x 10²³) of molecules. The numerical value of molar mass is identical to the molecular mass, but the unit is grams per mole (g/mol). In the case of KOH, the molar mass is approximately 56.11 g/mol.
This distinction is critical in stoichiometric calculations where we deal with macroscopic amounts of substances. Molar mass allows us to convert between the mass of a substance and the number of moles present.
Applications of KOH and the Importance of Knowing its Molecular Mass
KOH has a wide array of applications across various industries, and accurate knowledge of its molecular mass is essential in these contexts:
1. Chemical Reactions and Stoichiometry:
Knowing the molecular mass of KOH is vital for calculating the amounts of reactants and products in chemical reactions involving KOH. This is crucial for optimizing reaction yields, controlling reaction conditions, and ensuring safety.
2. Titrations and Acid-Base Chemistry:
KOH is a strong base commonly used in titrations to determine the concentration of acids. Precise calculations of its molecular mass are essential for accurate determination of acid concentrations.
3. Solution Preparation:
Preparing solutions of KOH with specific concentrations necessitates the accurate calculation of the mass required. This ensures the desired concentration is achieved and avoids potential errors in experiments or industrial processes.
4. Fertilizer Production:
KOH is used in the production of certain fertilizers. Knowing its molecular mass is important for determining the amount of KOH needed to create fertilizers with specific nutrient compositions.
5. Soap and Detergent Manufacturing:
KOH is a key ingredient in the saponification process of soap and detergent manufacturing. Accurate calculations involving its molecular mass are crucial for controlling the quality and properties of the final product.
6. Food Industry:
KOH, in controlled amounts, serves as a food additive and a pH regulator in certain food products. Accurate molecular mass determination is critical to maintaining quality and safety standards.
Potential Sources of Error and Precautions
While the calculation of KOH's molecular mass is relatively straightforward, several factors can introduce minor inaccuracies:
-
Isotopic Abundance: The atomic masses used are average values based on the natural abundance of isotopes. Variations in isotopic composition can lead to slight differences in the calculated molecular mass.
-
Rounding Errors: Rounding off atomic masses during the calculation can accumulate small errors. Using more significant figures in the atomic masses minimizes these errors.
-
Impurities: If the KOH sample contains impurities, the calculated molecular mass may not accurately represent pure KOH.
It's crucial to use a reliable periodic table with accurate atomic masses and to be mindful of potential rounding errors to minimize inaccuracies. For highly sensitive applications, more rigorous analytical techniques may be necessary to determine the precise molecular mass.
Advanced Calculations and Applications:
The basic calculation we've covered provides a fundamental understanding. However, more complex scenarios might require additional considerations:
-
Hydrates: KOH can exist as hydrates (e.g., KOH·H₂O). In such cases, you must include the mass of the water molecules in the calculation.
-
Isotopic Analysis: For applications requiring high precision, isotopic analysis can provide more accurate atomic masses, leading to a more precise molecular mass determination.
-
Spectroscopic Techniques: Techniques like mass spectrometry offer precise measurements of molecular mass and can be crucial in advanced research or quality control.
Conclusion
Calculating the molecular mass of KOH, or any compound, involves a simple yet fundamental process that depends on understanding the concept of atomic mass and utilizing the periodic table effectively. This seemingly simple calculation has profound implications in various scientific, industrial, and technological applications, highlighting the importance of mastering this basic yet crucial concept in chemistry. By understanding the principles involved and potential sources of error, one can confidently calculate molecular masses and apply this knowledge to a wide range of problems. Remember to always utilize the most up-to-date periodic table for the most accurate results. This comprehensive guide serves as a starting point for a deeper understanding of this vital concept in chemistry.
Latest Posts
Latest Posts
-
216 To The Power Of 1 3
Apr 08, 2025
-
What Is The Factors For 32
Apr 08, 2025
-
Number Of Valence Electrons For Boron
Apr 08, 2025
-
What Holds Two Strands Of Dna Together
Apr 08, 2025
-
What Happens To Atoms After A Chemical Change
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Molecular Mass Of Koh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.