Calculate The Mass Of Br 79
listenit
Mar 22, 2025 · 5 min read
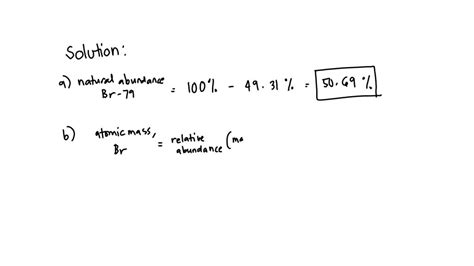
Table of Contents
Calculating the Mass of Br-79: A Deep Dive into Isotopes and Atomic Mass
Determining the mass of Br-79, a specific isotope of bromine, requires understanding the concepts of atomic mass, isotopes, and the use of the atomic mass unit (amu). This article will provide a comprehensive explanation, moving from fundamental principles to practical calculations, ensuring you have a solid grasp of this important concept in chemistry.
Understanding Isotopes and Atomic Mass
Before we delve into the specific calculation for Br-79, let's establish a firm foundation. Atoms of the same element can exist in different forms called isotopes. Isotopes have the same number of protons (defining the element) but differ in the number of neutrons in their nucleus. This difference in neutron number affects the atomic mass of the isotope.
- Atomic Number: The number of protons in an atom's nucleus. This defines the element. Bromine (Br) has an atomic number of 35.
- Mass Number: The total number of protons and neutrons in an atom's nucleus. This is often represented as a superscript before the element symbol (e.g., ⁷⁹Br).
- Atomic Mass Unit (amu): A unit of mass used to express the mass of atoms and molecules. It's defined as 1/12 the mass of a carbon-12 atom. Approximately, 1 amu is equal to 1.66 x 10⁻²⁴ grams.
Br-79, specifically, indicates a bromine isotope with a mass number of 79. This means it has 35 protons (because it's bromine) and 79 - 35 = 44 neutrons.
Calculating the Mass of Br-79 in amu
The mass of Br-79 in amu isn't simply 79 amu. While the mass number provides a close approximation, the actual mass is slightly different due to mass defect, a consequence of the strong nuclear force binding the protons and neutrons together. This mass defect is converted into binding energy according to Einstein's famous equation, E=mc².
Precise measurements using mass spectrometry reveal the actual atomic mass of Br-79 to be approximately 78.9183 amu. This value is a weighted average considering various factors like nuclear binding energy and the interaction between subatomic particles. You won't be calculating this value from scratch; it's determined experimentally.
The Significance of Average Atomic Mass
Bromine, like many elements, exists naturally as a mixture of isotopes. The most common isotopes are Br-79 and Br-81. The average atomic mass listed on the periodic table is a weighted average of the masses of these isotopes, taking into account their relative abundances.
The relative abundances of Br-79 and Br-81 are approximately 50.69% and 49.31%, respectively. This means that in a sample of bromine, about 50.69% of the atoms are Br-79, and the rest are Br-81.
To calculate the average atomic mass of bromine:
(Abundance of Br-79 x Mass of Br-79) + (Abundance of Br-81 x Mass of Br-81) = Average Atomic Mass of Br
(0.5069 x 78.9183 amu) + (0.4931 x 80.9163 amu) ≈ 79.904 amu
This average atomic mass (approximately 79.904 amu) is the value you'll find on the periodic table. It's crucial to remember that this is an average and doesn't represent the mass of any single bromine atom.
Converting amu to Grams
To express the mass of Br-79 in grams, we utilize the conversion factor mentioned earlier: 1 amu ≈ 1.66 x 10⁻²⁴ grams.
Mass of Br-79 in grams = Mass of Br-79 in amu x (1.66 x 10⁻²⁴ g/amu)
Mass of Br-79 in grams = 78.9183 amu x (1.66 x 10⁻²⁴ g/amu) ≈ 1.31 x 10⁻²² grams
This calculation shows that a single atom of Br-79 has an incredibly small mass.
Practical Applications and Further Considerations
Understanding the mass of isotopes, particularly Br-79, has several crucial applications in various scientific fields:
1. Mass Spectrometry:
Mass spectrometry is a powerful analytical technique that separates ions based on their mass-to-charge ratio. This allows for the precise determination of isotopic abundances and atomic masses, like that of Br-79.
2. Nuclear Chemistry and Physics:
The study of nuclear reactions and radioactive decay processes often involves precise knowledge of isotopic masses. This includes calculating the energy released or absorbed during nuclear transformations.
3. Chemical Kinetics and Reaction Rates:
Isotopic masses can subtly influence reaction rates, especially in systems involving heavy isotopes.
4. Geochemistry and Environmental Science:
Isotopic ratios are used extensively in geochemistry to determine the age of rocks and minerals and to trace the movement of elements in the environment. Bromine isotopes, including Br-79, can be valuable indicators in various environmental studies.
5. Medical Imaging and Treatment:
Specific isotopes of elements are employed in medical imaging techniques (e.g., PET scans) and radiation therapy. Understanding the mass and properties of these isotopes is crucial for their effective application.
Conclusion: Mastering Isotope Mass Calculations
Calculating the mass of Br-79, while seemingly straightforward, requires a thorough understanding of atomic structure, isotopes, and the intricacies of atomic mass units. This detailed explanation has gone beyond a simple calculation, providing a comprehensive overview of the underlying principles and their practical implications. Remember that the mass of 78.9183 amu is an experimentally determined value. While the mass number (79) provides a useful approximation, the precise mass is subtly different due to the complex interplay of nuclear forces. This knowledge is fundamental for anyone pursuing studies in chemistry, physics, or related fields. By understanding the concepts outlined here, you’re well-equipped to tackle more complex problems involving isotopes and their masses.
Latest Posts
Latest Posts
-
30 Of What Number Is 15
Mar 23, 2025
-
9 Is What Percent Of 90
Mar 23, 2025
-
What Are The Most Reactive Nonmetals
Mar 23, 2025
-
What Is 1 2 Kilos In Pounds
Mar 23, 2025
-
What Is The Square Root Of 83
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Mass Of Br 79 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
