Burning Wood Chemical Or Physical Change
listenit
Mar 18, 2025 · 6 min read
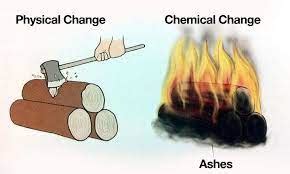
Table of Contents
Burning Wood: A Chemical Change Unveiled
Burning wood is a quintessential example of a chemical change, a process that fundamentally alters the molecular structure of a substance, resulting in the formation of entirely new substances with different properties. Unlike physical changes, which only affect the form or appearance of a substance without changing its chemical composition, burning wood involves a complex series of chemical reactions that transform the wood into ash, gases, and energy. Understanding this process requires delving into the chemical makeup of wood and the intricate reactions involved in combustion.
The Composition of Wood: A Complex Mixture
Before exploring the chemical changes involved in burning wood, let's examine the substance itself. Wood is not a homogenous material; instead, it's a complex composite primarily consisting of three main components:
1. Cellulose: The Structural Backbone
Cellulose forms the structural framework of wood, accounting for approximately 40-50% of its dry weight. It's a long-chain polymer composed of glucose units linked together, giving wood its strength and rigidity. Cellulose is a carbohydrate, a class of organic compounds containing carbon, hydrogen, and oxygen.
2. Hemicellulose: A Supporting Role
Hemicellulose, another carbohydrate, makes up around 20-35% of wood's dry weight. Unlike cellulose, hemicellulose has a more complex and branched structure, consisting of various sugars. It acts as a supporting component within the wood structure, contributing to its overall properties.
3. Lignin: The Glue that Holds it Together
Lignin, a complex polymer with a high molecular weight, accounts for 15-30% of wood's dry weight. It acts as a binding agent, connecting the cellulose and hemicellulose fibers, providing stiffness and water resistance to the wood. Lignin's chemical structure is far more complex and varied than cellulose or hemicellulose, contributing significantly to the diversity of chemical reactions during combustion.
Minor Components: The Subtle Influences
Besides these major components, wood also contains small amounts of extractives, such as resins, tannins, and oils. These components influence the wood's color, odor, and other properties, but they play a relatively minor role in the overall combustion process. However, their presence can subtly alter the nature of the byproducts.
The Chemistry of Burning Wood: A Cascade of Reactions
The burning of wood, or combustion, is a highly exothermic reaction, meaning it releases a significant amount of heat. It's a complex process involving multiple steps and numerous chemical reactions. The overall reaction can be summarized as the oxidation of wood components, primarily cellulose, hemicellulose, and lignin, in the presence of oxygen.
1. Ignition: The Starting Point
The process begins with ignition, where the wood is heated to its ignition temperature. This typically requires a significant input of energy, whether through a flame, spark, or friction. The initial heat overcomes the activation energy barrier, initiating the breakdown of wood components.
2. Pyrolysis: The Breakdown of Wood
Once the ignition temperature is reached, pyrolysis occurs. Pyrolysis is the thermal decomposition of wood in the absence of oxygen. The heat breaks down the cellulose, hemicellulose, and lignin molecules into smaller, volatile organic compounds (VOCs). These VOCs include a wide array of substances, such as:
- Gases: Carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other hydrocarbons.
- Volatile liquids: Water (H2O), methanol (CH3OH), acetic acid (CH3COOH), and other organic acids.
- Char: A carbon-rich residue that remains after the volatile compounds have been released.
This stage is crucial because it transforms the complex structure of wood into simpler molecules that can readily react with oxygen.
3. Combustion: The Reaction with Oxygen
The VOCs released during pyrolysis then react with oxygen in the air. This is the combustion phase, an exothermic reaction generating heat and light. The complete combustion of these VOCs ideally produces carbon dioxide (CO2) and water (H2O), along with energy in the form of heat and light.
However, complete combustion rarely occurs, especially in the case of burning wood. Incomplete combustion can lead to the formation of other products, including:
- Carbon Monoxide (CO): A highly toxic and colorless gas.
- Soot (Elemental Carbon): Fine particles of carbon that contribute to air pollution.
- Unburnt Hydrocarbons: These contribute to air pollution and smog.
The presence of these incomplete combustion products highlights the chemical complexity of burning wood and the environmental considerations involved.
4. Ash: The Residue
After the volatile components have been consumed, the remaining material is ash. This residue is primarily composed of inorganic minerals present in the wood, such as potassium, calcium, and magnesium. The composition of ash can provide insight into the type of wood and its mineral content.
Evidence of Chemical Change: Irreversible Transformation
The burning of wood clearly demonstrates several features characteristic of a chemical change:
- Formation of new substances: The original wood is transformed into completely different substances: ash, gases (CO2, CO, etc.), and water vapor. These substances have different chemical properties and compositions compared to the original wood.
- Irreversibility: The process cannot be reversed easily. You cannot simply reassemble the ash, gases, and water vapor to recreate the original piece of wood.
- Energy change: The combustion process releases a significant amount of energy in the form of heat and light, indicating a substantial chemical transformation.
- Change in properties: The original wood's physical and chemical properties (e.g., color, texture, flammability) are permanently altered.
Distinguishing from Physical Changes
It's crucial to differentiate chemical changes, like burning wood, from physical changes. Physical changes only alter the form or appearance of a substance without changing its chemical composition. For example, cutting, sawing, or grinding wood are physical changes because they alter the wood's shape and size but don't change its chemical composition. The chemical bonds within the wood molecules remain intact. Melting ice into water is another example of a physical change; the water molecules remain unchanged.
Burning wood, however, creates new chemical substances, fundamentally altering its chemical composition, clearly indicating a chemical change.
The Environmental Impact: A Crucial Consideration
While burning wood can provide energy, it's essential to acknowledge its environmental impact. Incomplete combustion produces harmful pollutants, including carbon monoxide and particulate matter, contributing to air pollution and respiratory problems. The release of carbon dioxide also contributes to climate change. Therefore, responsible and sustainable wood burning practices are crucial to minimize its negative environmental consequences.
Conclusion: A Complex Chemical Process with Real-World Implications
Burning wood is a fascinating and complex chemical process involving a cascade of reactions that transform the wood's chemical structure. Understanding this process, from the composition of wood to the chemical reactions involved, sheds light on the nature of chemical changes and their real-world implications. While burning wood offers energy, it also comes with significant environmental considerations, requiring responsible practices to mitigate its negative impacts. The contrast between burning wood (a chemical change) and simpler physical alterations highlights the fundamental differences between these two types of transformations in matter. Recognizing this distinction is crucial for appreciating the broader principles of chemistry and the intricate world of chemical reactions.
Latest Posts
Latest Posts
-
What Is The Percentage Of 1 12
Mar 18, 2025
-
Product Of Nacl Hn03 And Na2co3
Mar 18, 2025
-
What Is 0 01 As A Fraction
Mar 18, 2025
-
What Are The Missing Reasons In The Proof
Mar 18, 2025
-
3 6 4 8 6 12 10
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Burning Wood Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
