Bronsted Lowry Acid Vs Lewis Acid
listenit
Mar 15, 2025 · 6 min read
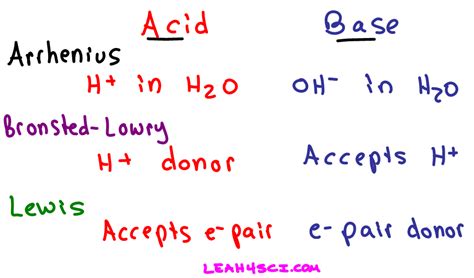
Table of Contents
Brønsted-Lowry Acid vs. Lewis Acid: A Comprehensive Comparison
Understanding the difference between Brønsted-Lowry and Lewis acids is crucial for a solid grasp of acid-base chemistry. While both definitions describe substances that act as acids, they differ significantly in their scope and mechanism. This comprehensive guide will delve into the nuances of each definition, highlighting their similarities, differences, and providing examples to solidify your understanding.
Brønsted-Lowry Acid-Base Theory: A Proton's Perspective
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, focuses on the transfer of protons (H⁺). According to this theory:
- A Brønsted-Lowry acid is a proton donor. It's a substance that donates a proton (H⁺) to another substance.
- A Brønsted-Lowry base is a proton acceptor. It's a substance that accepts a proton (H⁺) from another substance.
This theory elegantly explains many acid-base reactions, particularly those involving aqueous solutions. The reaction always involves a conjugate acid-base pair. When an acid donates a proton, it forms its conjugate base, which is capable of accepting a proton to revert back to the original acid. Similarly, when a base accepts a proton, it forms its conjugate acid.
Examples of Brønsted-Lowry Acid-Base Reactions:
1. The reaction between hydrochloric acid (HCl) and water (H₂O):
HCl (acid) + H₂O (base) ⇌ H₃O⁺ (conjugate acid) + Cl⁻ (conjugate base)
Here, HCl donates a proton to H₂O, forming the hydronium ion (H₃O⁺) and the chloride ion (Cl⁻). HCl is the acid, H₂O is the base, H₃O⁺ is the conjugate acid, and Cl⁻ is the conjugate base.
2. The reaction between ammonia (NH₃) and water (H₂O):
NH₃ (base) + H₂O (acid) ⇌ NH₄⁺ (conjugate acid) + OH⁻ (conjugate base)
In this reaction, water acts as an acid, donating a proton to ammonia, forming the ammonium ion (NH₄⁺) and the hydroxide ion (OH⁻). Notice that water can act as both an acid and a base, depending on the reaction. This is known as amphiprotic behavior.
Strengths and Limitations of Brønsted-Lowry Theory:
The Brønsted-Lowry theory is a powerful tool for understanding many acid-base reactions. However, it has limitations:
- It doesn't explain all acid-base reactions. Some reactions involve acid-base behavior without any proton transfer.
- It focuses solely on proton transfer. This limits its applicability to reactions not involving protons.
Lewis Acid-Base Theory: A Broader Perspective
Gilbert N. Lewis proposed a more general definition of acids and bases in 1923, focusing on the donation and acceptance of electron pairs. This theory significantly expands the scope of acid-base chemistry.
- A Lewis acid is an electron-pair acceptor. It's a substance that can accept a pair of electrons to form a coordinate covalent bond.
- A Lewis base is an electron-pair donor. It's a substance that can donate a pair of electrons to form a coordinate covalent bond.
This definition encompasses a wider range of substances than the Brønsted-Lowry definition. Any substance with an empty orbital capable of accepting an electron pair can act as a Lewis acid, and any substance with a lone pair of electrons can act as a Lewis base.
Examples of Lewis Acid-Base Reactions:
1. The reaction between boron trifluoride (BF₃) and ammonia (NH₃):
BF₃ (Lewis acid) + NH₃ (Lewis base) → F₃B←NH₃
Here, BF₃, with an incomplete octet, accepts a lone pair of electrons from the nitrogen atom in NH₃, forming a coordinate covalent bond. BF₃ is the Lewis acid, and NH₃ is the Lewis base. Note that no proton transfer occurs in this reaction.
2. The reaction between silver ion (Ag⁺) and ammonia (NH₃):
Ag⁺ (Lewis acid) + 2NH₃ (Lewis base) → [Ag(NH₃)₂]⁺
The silver ion, with an empty orbital, accepts a lone pair of electrons from each ammonia molecule, forming a complex ion. Again, no proton transfer occurs.
3. The reaction between aluminum chloride (AlCl₃) and chloride ion (Cl⁻):
AlCl₃ (Lewis acid) + Cl⁻ (Lewis base) → AlCl₄⁻
Aluminum chloride, having an incomplete octet, accepts a lone pair of electrons from the chloride ion, forming the tetrachloroaluminate ion.
Strengths and Advantages of Lewis Theory:
The Lewis theory offers several advantages over the Brønsted-Lowry theory:
- It's more general. It encompasses a wider range of reactions, including those without proton transfer.
- It provides a more comprehensive understanding of acid-base behavior. It explains reactions involving metal ions, coordination compounds, and many organic reactions.
- It helps predict reaction outcomes. Understanding electron pair donation and acceptance helps predict the formation of new bonds and the stability of resulting products.
Comparing Brønsted-Lowry and Lewis Acids: A Side-by-Side Analysis
| Feature | Brønsted-Lowry Acid | Lewis Acid |
|---|---|---|
| Definition | Proton (H⁺) donor | Electron-pair acceptor |
| Mechanism | Proton transfer | Electron pair donation and acceptance |
| Scope | Limited to reactions involving proton transfer | Broader; encompasses many reactions without proton transfer |
| Examples | HCl, H₂SO₄, CH₃COOH, H₂O (as acid) | BF₃, AlCl₃, Fe³⁺, CO₂ |
| Conjugate Pairs | Forms conjugate base upon proton donation | No concept of conjugate pairs in the same sense |
Bridging the Gap: The Relationship Between the Two Theories
It's important to understand that the Lewis theory is a more encompassing theory. All Brønsted-Lowry acids are also Lewis acids, but not all Lewis acids are Brønsted-Lowry acids.
A Brønsted-Lowry acid donates a proton, which inherently involves accepting an electron pair from the base. Therefore, it fulfills the criteria of a Lewis acid. However, many substances can act as Lewis acids without donating protons, as illustrated by the examples above (BF₃, AlCl₃, Ag⁺).
Applications and Importance
Understanding both Brønsted-Lowry and Lewis acid-base theories is crucial in various fields:
- Inorganic Chemistry: Describing the behavior of metal ions and coordination complexes.
- Organic Chemistry: Understanding reaction mechanisms, such as nucleophilic additions and electrophilic substitutions.
- Biochemistry: Explaining the role of acids and bases in biological systems, such as enzyme catalysis and protein folding.
- Analytical Chemistry: Developing titrations and other analytical techniques for determining acid-base concentrations.
- Industrial Chemistry: Designing catalysts and controlling reaction conditions in industrial processes.
Conclusion: A Unified Perspective
While the Brønsted-Lowry theory provides a valuable framework for understanding many acid-base reactions involving proton transfer, the Lewis theory offers a broader, more comprehensive perspective encompassing a wider range of chemical interactions. By understanding both theories, we gain a deeper appreciation of the complexities and versatility of acid-base chemistry, allowing us to predict and explain a greater variety of chemical phenomena across diverse fields of study. The Lewis theory effectively expands upon the Brønsted-Lowry theory, providing a more complete and nuanced understanding of acid-base interactions in the chemical world.
Latest Posts
Latest Posts
-
Integral Of Tan 2x Sec 2x
Mar 15, 2025
-
How Do I Convert Torr To Atm
Mar 15, 2025
-
What Is The Lowest Common Multiple Of 5 And 7
Mar 15, 2025
-
What Is The Percent Of 9 25
Mar 15, 2025
-
What Is 4 5 In Fraction Form
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Bronsted Lowry Acid Vs Lewis Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
