Boiling Water Is Physical Or Chemical Change
listenit
Mar 17, 2025 · 5 min read
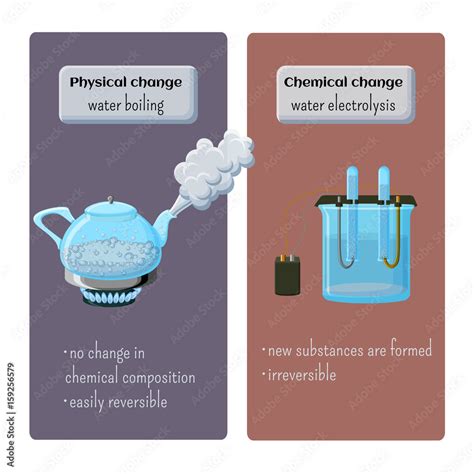
Table of Contents
Is Boiling Water a Physical or Chemical Change? A Deep Dive
The question of whether boiling water represents a physical or chemical change is a common one, especially in science classes. While seemingly simple, it delves into the fundamental concepts of matter and its transformations. The answer, however, isn't a straightforward "yes" or "no," but rather a nuanced exploration of the processes involved. Understanding this requires a solid grasp of the definitions of physical and chemical changes and a detailed look at what happens to water molecules during boiling.
Understanding Physical and Chemical Changes
Before diving into the specifics of boiling water, let's establish clear definitions:
Physical Change: A physical change alters the form or appearance of a substance but doesn't change its chemical composition. The molecules remain the same; only their arrangement or state of matter changes. Examples include melting ice, tearing paper, or dissolving sugar in water. The substance can often be recovered in its original form through a physical process.
Chemical Change: A chemical change, also known as a chemical reaction, involves the rearrangement of atoms to form new substances with different chemical properties. The original substance is transformed into something fundamentally different. Examples include burning wood, rusting iron, or cooking an egg. The original substance cannot be easily recovered through simple physical means.
The Boiling Process: A Detailed Look
When water boils, it transitions from its liquid state to its gaseous state, a process called vaporization. This happens when the water molecules absorb enough energy (heat) to overcome the intermolecular forces holding them together in the liquid phase. These forces, primarily hydrogen bonds, are relatively strong in water, explaining its relatively high boiling point.
Molecular Level Changes During Boiling
At the molecular level, the process of boiling involves:
- Increased Kinetic Energy: As heat is added, the water molecules gain kinetic energy, moving faster and colliding more frequently.
- Overcoming Intermolecular Forces: With enough kinetic energy, the molecules can overcome the attractive forces (hydrogen bonds) holding them together in the liquid phase.
- Phase Transition: Once the molecules have sufficient energy, they escape the liquid surface and enter the gaseous phase as water vapor (steam). This is why we see bubbles forming and rising to the surface during boiling.
- No Change in Molecular Structure: Crucially, the water molecules themselves (H₂O) remain unchanged. They are still composed of two hydrogen atoms and one oxygen atom covalently bonded together.
Observable Changes During Boiling
While the molecular structure remains the same, several observable changes occur during boiling:
- Change in State: The most obvious change is the transition from liquid water to water vapor (steam).
- Temperature Remains Constant: At a constant pressure (e.g., atmospheric pressure), the temperature of boiling water remains constant at 100°C (212°F). This is because the added heat energy is used to overcome the intermolecular forces, not to increase the kinetic energy of the molecules.
- Volume Increase: Water vapor occupies a significantly larger volume than liquid water due to the increased distance between the molecules in the gaseous phase.
- Change in Density: Water vapor is much less dense than liquid water.
Is Boiling Water a Physical or Chemical Change? The Verdict
Given the analysis above, the conclusion is that boiling water is a physical change. Although there are observable changes in state, volume, and density, the fundamental chemical composition of the water molecules (H₂O) remains unchanged. No new substances are formed. If you were to condense the steam back into liquid water, you would recover the original substance.
Common Misconceptions
Several misconceptions surrounding boiling water often lead to confusion:
- Confusion with Decomposition: Some might confuse boiling with decomposition, where a substance breaks down into simpler components. Boiling is not decomposition; it's a phase transition.
- Ignoring the Subtleties: Focusing solely on the observable changes (state change, volume increase) without considering the molecular level changes can lead to incorrect conclusions.
- Oversimplification: The seemingly simple act of boiling water involves a complex interplay of forces and energy transfer at the molecular level.
Extending the Understanding: Related Concepts
The concept of boiling water as a physical change helps illustrate several related scientific principles:
- Phase Transitions: Boiling is one example of a phase transition, where a substance changes from one state of matter to another (solid, liquid, gas, plasma). Melting, freezing, sublimation, and deposition are other examples.
- Intermolecular Forces: The strength of intermolecular forces significantly impacts the boiling point of a substance. Water's strong hydrogen bonds account for its relatively high boiling point compared to other similar-sized molecules.
- Kinetic Molecular Theory: This theory explains the behavior of matter in terms of the motion and energy of its constituent particles. It explains why increasing the temperature of water increases the kinetic energy of its molecules, eventually leading to boiling.
- Heat Capacity and Latent Heat: The amount of heat required to raise the temperature of a substance and the amount of heat required to change its state (latent heat) are important concepts related to boiling.
Practical Applications and Real-World Examples
The understanding that boiling water is a physical change has numerous practical implications:
- Cooking: Boiling water is crucial for many cooking methods, where the heat is used to transfer energy to food items, not to chemically alter the water.
- Sterilization: Boiling water is used for sterilization because the high temperature kills microorganisms, not because it changes the chemical composition of the water.
- Distillation: Distillation is a purification method that relies on the physical change of boiling and condensation to separate components of a mixture.
- Power Generation: Steam, generated by boiling water, is used in power plants to drive turbines and generate electricity.
Conclusion
In conclusion, boiling water is unequivocally a physical change. While significant changes in the observable properties of water occur during boiling, the chemical composition of the water molecules remains unchanged. This seemingly simple process provides a fascinating glimpse into the fundamental principles of matter, energy, and phase transitions, showcasing the importance of understanding both macroscopic and microscopic perspectives in science. The ability to differentiate between physical and chemical changes is essential for a complete understanding of numerous scientific concepts and practical applications.
Latest Posts
Latest Posts
-
How Many Meters In 100 Cm
Mar 17, 2025
-
How Many 1 3 Are In 1 Cup
Mar 17, 2025
-
Greatest Common Factor Of 18 And 24
Mar 17, 2025
-
30 Of What Number Is 27
Mar 17, 2025
-
Is Rate Of Change The Same As Slope
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Is Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
