Boiling Point Is A Chemical Property
listenit
Mar 29, 2025 · 6 min read
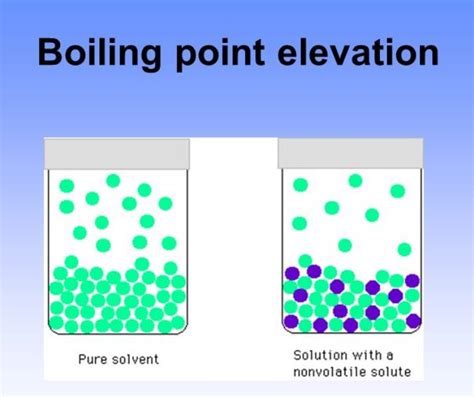
Table of Contents
Boiling Point: A Fundamental Chemical Property
Boiling point, a seemingly simple concept, is actually a crucial and defining chemical property. Understanding its significance unlocks deeper insights into the nature of matter and its interactions. This article delves into the intricacies of boiling point, explaining its definition, the factors that influence it, its importance in various scientific fields, and its applications in everyday life.
Defining Boiling Point: More Than Just Bubbles
The boiling point of a substance is defined as the temperature at which its vapor pressure equals the external pressure surrounding the liquid. In simpler terms, it's the temperature at which a liquid transitions to a gas phase throughout its entire volume. This is different from evaporation, which occurs at the surface of a liquid at any temperature. Boiling is characterized by the rapid formation of vapor bubbles within the liquid, rising to the surface and escaping.
The most commonly cited boiling point is the normal boiling point, which is the temperature at which the vapor pressure of the liquid equals one standard atmosphere (101.325 kPa or 760 mmHg). This is the boiling point we typically encounter in everyday life and find tabulated in reference materials. However, it's crucial to remember that the boiling point is pressure-dependent. At higher pressures, the boiling point increases, while at lower pressures, it decreases. This is why water boils at a lower temperature at high altitudes where atmospheric pressure is reduced.
Factors Influencing Boiling Point: Intermolecular Forces and Molecular Weight
Several factors influence a substance's boiling point. The primary factors are:
1. Intermolecular Forces: The Glue Holding Molecules Together
The strength of intermolecular forces (IMFs) between molecules significantly impacts the boiling point. Stronger IMFs require more energy to overcome the attractive forces between molecules, resulting in a higher boiling point. The main types of IMFs are:
-
London Dispersion Forces (LDFs): Present in all molecules, LDFs are weak, temporary attractions caused by instantaneous fluctuations in electron distribution. Larger molecules with more electrons generally exhibit stronger LDFs.
-
Dipole-Dipole Interactions: Occur between polar molecules possessing permanent dipoles. These interactions are stronger than LDFs and lead to higher boiling points compared to nonpolar molecules of similar size.
-
Hydrogen Bonding: A special type of dipole-dipole interaction involving hydrogen atoms bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine. Hydrogen bonds are considerably stronger than typical dipole-dipole interactions and result in significantly higher boiling points. Water's exceptionally high boiling point is a direct consequence of its strong hydrogen bonding network.
2. Molecular Weight: Size Matters
Higher molecular weight generally correlates with a higher boiling point. Larger molecules have more electrons, leading to stronger LDFs, thus requiring more energy to break these attractions and transition to the gaseous phase. This effect is particularly noticeable in nonpolar substances where LDFs are the dominant IMFs.
3. Molecular Shape and Branching: Packing Efficiency
Molecular shape and branching affect the packing efficiency of molecules in the liquid phase. Linear molecules tend to pack more efficiently than branched molecules, resulting in stronger IMFs and higher boiling points. Branched molecules have a lower surface area for interaction, leading to weaker IMFs and lower boiling points compared to their linear counterparts.
4. External Pressure: The Environmental Impact
As mentioned earlier, external pressure significantly influences the boiling point. Reducing the external pressure lowers the boiling point, while increasing the pressure raises it. This is a fundamental principle utilized in pressure cookers, where increased pressure allows for higher cooking temperatures, thus shortening cooking times.
Boiling Point: A Tool in Various Scientific Fields
Boiling point determination plays a crucial role in various scientific fields, including:
1. Chemistry: Identification and Purification
Boiling point is a characteristic property used to identify unknown substances and assess the purity of compounds. A discrepancy between the observed boiling point and the literature value suggests the presence of impurities. Fractional distillation, a technique based on the differences in boiling points, is employed to separate mixtures of liquids with different boiling points.
2. Pharmacy and Medicine: Drug Characterization and Formulation
In the pharmaceutical industry, boiling point is a critical parameter for characterizing drugs and formulating medications. It helps determine the appropriate solvents for drug dissolution, purification, and stability. The boiling point also impacts the design of drug delivery systems.
3. Materials Science: Material Selection and Processing
Boiling point is a vital property considered when selecting and processing materials. The boiling points of solvents used in material synthesis and processing determine their suitability for specific applications. The boiling point also influences the thermal stability of materials.
4. Environmental Science: Pollution Monitoring and Remediation
Boiling point can be used to characterize pollutants and aid in environmental remediation. Understanding the boiling points of volatile organic compounds (VOCs) is crucial for developing effective air purification strategies.
5. Food Science: Cooking and Preservation
Boiling point directly impacts cooking processes and food preservation techniques. The boiling point of water determines the maximum temperature achievable during boiling, influencing cooking times and food textures. Pressure canning utilizes the principle of elevated boiling point to achieve sterilization at higher temperatures.
Boiling Point in Everyday Life: Beyond the Chemistry Lab
The concept of boiling point is not confined to the laboratory; it plays a significant role in many aspects of our daily lives:
-
Cooking: We utilize the boiling point of water to cook various foods, from pasta to vegetables. The precise temperature of boiling water determines cooking times and food textures.
-
Cleaning: Many household cleaning solutions utilize boiling water to enhance their cleaning power. The high temperature helps to dissolve and remove grease and grime.
-
Beverages: Brewing coffee and tea involves precise control of water temperature to extract optimal flavor compounds. The boiling point of water dictates the maximum temperature achievable using conventional methods.
-
Altitude and Cooking: At higher altitudes where atmospheric pressure is lower, water boils at a lower temperature. This requires adjusting cooking times to ensure food is cooked properly.
-
Pressure Cookers: Pressure cookers use increased pressure to elevate the boiling point of water, allowing for higher cooking temperatures and faster cooking times.
Advanced Concepts and Applications: Beyond the Basics
Beyond the fundamental principles discussed above, several advanced concepts and applications relate to boiling point:
-
Superheating: This occurs when a liquid is heated above its boiling point without boiling. This is possible if there are no nucleation sites (surface imperfections or impurities) for bubble formation. Superheating can be dangerous, as the sudden, explosive boiling can cause significant hazards.
-
Boiling Point Elevation: Adding a non-volatile solute to a solvent increases the boiling point of the solution. This phenomenon, explained by colligative properties, is relevant in various applications, such as antifreeze formulations.
-
Clausius-Clapeyron Equation: This equation describes the relationship between the vapor pressure of a liquid and its temperature. It's crucial in determining the boiling point at different pressures.
-
Phase Diagrams: Phase diagrams visually represent the states of matter of a substance under different conditions of temperature and pressure. The boiling point is a key feature on a phase diagram, marking the boundary between the liquid and gas phases.
Conclusion: The Unsung Hero of Chemistry
Boiling point, while often overlooked, is a fundamental chemical property with far-reaching implications. Understanding its definition, the factors that influence it, and its applications across various scientific fields and everyday life equips us with valuable insights into the behavior of matter and its interactions. From identifying unknown compounds to designing efficient cooking methods, the boiling point remains an essential concept in both scientific research and practical applications. Its continued study offers valuable opportunities for advancements in various technological and scientific domains.
Latest Posts
Latest Posts
-
40 Is 32 Percent Of What Number
Mar 31, 2025
-
What Is The Highest Point Of A Transverse Wave Called
Mar 31, 2025
-
Diameter Of The Solar System In Light Years
Mar 31, 2025
-
48 Of 60 Is What Percent
Mar 31, 2025
-
Is Adenine A Purine Or Pyrimidine
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Boiling Point Is A Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
