Balanced Equation For Copper And Nitric Acid
listenit
Apr 06, 2025 · 5 min read
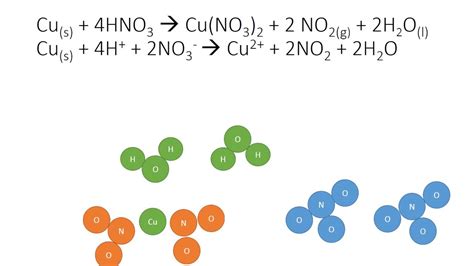
Table of Contents
The Balanced Equation for Copper and Nitric Acid: A Deep Dive into Redox Reactions
The reaction between copper (Cu) and nitric acid (HNO₃) is a classic example of a redox reaction, a chemical process involving the transfer of electrons between species. Understanding this reaction requires delving into its intricacies, encompassing the balanced chemical equations, the influencing factors, and the diverse applications stemming from this fundamental chemical process. This detailed exploration will provide a comprehensive understanding, suitable for students and enthusiasts alike.
Understanding the Reaction: A Redox Perspective
Copper, a transition metal, readily reacts with strong oxidizing agents like nitric acid. Nitric acid, a strong acid, acts as both an acid and an oxidizing agent, meaning it donates protons (H⁺) and accepts electrons. In this reaction, copper is oxidized (loses electrons), while nitrogen in nitric acid is reduced (gains electrons). The outcome is the formation of copper(II) ions, nitrogen oxides (the specific oxide depends on the concentration of the nitric acid), and water.
The reaction doesn't proceed in a single step; rather, it's a complex process involving several intermediate steps. The specific products formed depend strongly on the concentration of the nitric acid.
Concentrated Nitric Acid Reaction
When copper reacts with concentrated nitric acid (typically 16M or higher), the primary nitrogen oxide formed is nitrogen dioxide (NO₂), a brown gas. The balanced equation for this reaction is:
Cu(s) + 4HNO₃(conc.) → Cu(NO₃)₂(aq) + 2NO₂(g) + 2H₂O(l)
Let's break down this equation:
- Cu(s): Solid copper is the reactant, represented in its elemental form.
- 4HNO₃(conc.): Concentrated nitric acid, acting as both an acid and oxidizing agent. The coefficient 4 indicates the stoichiometric ratio required for complete reaction.
- Cu(NO₃)₂(aq): Copper(II) nitrate, a soluble salt, is formed as a product. The "(aq)" indicates it's dissolved in aqueous solution.
- 2NO₂(g): Nitrogen dioxide, a reddish-brown gas, is another product. The "(g)" signifies its gaseous state.
- 2H₂O(l): Water is also formed as a product. The "(l)" indicates its liquid state.
This equation showcases the oxidation of copper (Cu → Cu²⁺ + 2e⁻) and the reduction of nitrogen (+5 in HNO₃ to +4 in NO₂). The electrons released by copper are accepted by nitrogen atoms in nitric acid.
Dilute Nitric Acid Reaction
In contrast, when copper reacts with dilute nitric acid (typically 6M or less), the primary nitrogen oxide produced is nitric oxide (NO), a colorless gas. The balanced equation is more complex and can be represented as:
3Cu(s) + 8HNO₃(dil.) → 3Cu(NO₃)₂(aq) + 2NO(g) + 4H₂O(l)
Again, let's analyze the equation:
- 3Cu(s): Three moles of solid copper react.
- 8HNO₃(dil.): Eight moles of dilute nitric acid are needed for the complete reaction.
- 3Cu(NO₃)₂(aq): Three moles of copper(II) nitrate are produced.
- 2NO(g): Two moles of nitric oxide, a colorless gas, are formed.
- 4H₂O(l): Four moles of water are generated.
Note that the oxidation of copper remains the same (Cu → Cu²⁺ + 2e⁻), but the reduction of nitrogen changes (+5 in HNO₃ to +2 in NO). This difference highlights the dependence of the reaction's outcome on the nitric acid concentration. The lower concentration limits the oxidizing power of nitric acid, leading to a less oxidized nitrogen product (NO instead of NO₂).
Factors Influencing the Reaction
Several factors influence the reaction's rate and the specific products formed:
Concentration of Nitric Acid:
As already discussed, the concentration of nitric acid is crucial. Concentrated acid leads to the formation of NO₂, while dilute acid produces NO. This is because a higher concentration provides a greater availability of oxidizing agents, driving the reaction towards a more complete oxidation of copper and a higher oxidation state for nitrogen.
Temperature:
Increasing the temperature generally accelerates the reaction rate. Higher temperatures increase the kinetic energy of the reacting molecules, leading to more frequent and effective collisions.
Surface Area of Copper:
A larger surface area of copper metal increases the reaction rate. More surface area provides more contact points for the nitric acid to react, accelerating the electron transfer process. Finely divided copper will react more rapidly than a single, large piece of copper.
Presence of Other Substances:
The presence of other substances can either catalyze or inhibit the reaction. Some substances might increase the rate of electron transfer, while others might hinder the reaction by forming protective layers on the copper surface.
Applications and Significance
The reaction between copper and nitric acid has several important applications and implications:
Synthesis of Copper(II) Nitrate:
This reaction serves as a common method for preparing copper(II) nitrate, a crucial compound in various applications, including:
- Chemical synthesis: As a reagent in various chemical reactions.
- Electroplating: Used in the electroplating of copper.
- Catalysis: Acts as a catalyst in some organic reactions.
Production of Nitrogen Oxides:
The reaction produces nitrogen oxides (NO and NO₂), which are important in various industrial processes and have environmental implications. These gases contribute to air pollution and play a role in the formation of acid rain and smog.
Demonstrating Redox Reactions:
The reaction is frequently used in educational settings to illustrate redox reactions and the concepts of oxidation and reduction. The visible changes (e.g., the dissolution of copper and the formation of brown NO₂ gas) make it a visually engaging demonstration.
Safety Precautions
Working with nitric acid requires careful attention to safety protocols. Nitric acid is a corrosive and strong oxidizing agent. Always wear appropriate personal protective equipment (PPE), including gloves, safety goggles, and a lab coat. Conduct the experiment in a well-ventilated area, as the nitrogen oxides produced are toxic. Dispose of the waste products according to appropriate safety regulations.
Conclusion: A Multifaceted Reaction
The reaction between copper and nitric acid, though seemingly simple at first glance, offers a rich tapestry of chemical principles. Understanding the balanced equations, the factors influencing the reaction, and its practical applications provides valuable insight into redox chemistry and its significance in diverse scientific fields and industrial processes. This reaction serves as a compelling example of how seemingly straightforward chemical processes can unveil a deeper understanding of complex chemical transformations. Further exploration into the kinetics and thermodynamics of this reaction can reveal even more about its intricate mechanisms and provide a platform for further scientific investigation. Remember always to prioritize safety when conducting experiments involving strong acids and oxidizing agents.
Latest Posts
Latest Posts
-
A Rapid Automatic Response To A Stimulus
Apr 07, 2025
-
What Unit Is Pressure Measured In
Apr 07, 2025
-
How Much Is Half Of 1 1 2 Cups
Apr 07, 2025
-
Unit Circle Sin Cos Tan Csc Sec Cot
Apr 07, 2025
-
Balance The Equation N2 H2 Nh3
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Copper And Nitric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
