Are All Ionic Compounds Soluble In Water
listenit
Mar 19, 2025 · 6 min read
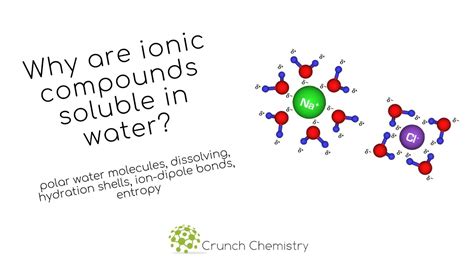
Table of Contents
Are All Ionic Compounds Soluble in Water? A Deep Dive into Solubility
The simple answer is no, not all ionic compounds are soluble in water. While many ionic compounds readily dissolve in water, a significant number are insoluble or only slightly soluble. Understanding the factors that govern the solubility of ionic compounds in water is crucial in various fields, from chemistry and environmental science to medicine and engineering. This article will delve into the intricacies of ionic compound solubility, exploring the underlying principles, influencing factors, and practical implications.
Understanding Solubility: A Molecular Perspective
Solubility refers to the ability of a substance to dissolve in a solvent, forming a homogeneous mixture called a solution. In the context of ionic compounds in water, solubility depends on the interplay between the attractive forces within the ionic compound itself (lattice energy) and the attractive forces between the ions and water molecules (hydration energy).
Lattice Energy: The Strength of the Crystal
Ionic compounds exist as crystalline solids, where positive and negative ions are held together by strong electrostatic forces, forming a three-dimensional lattice. The energy required to completely separate one mole of an ionic compound into its gaseous ions is called the lattice energy. The higher the lattice energy, the stronger the ionic bonds, and consequently, the harder it is to break apart the crystal structure. Factors contributing to high lattice energy include:
-
Charge of the ions: Higher charges on the ions lead to stronger electrostatic attractions and thus higher lattice energy. For example, MgO (Mg²⁺ and O²⁻) has a significantly higher lattice energy than NaCl (Na⁺ and Cl⁻).
-
Size of the ions: Smaller ions lead to higher lattice energy because the electrostatic forces are stronger at shorter distances. LiF has a higher lattice energy than CsI due to the smaller size of Li⁺ and F⁻ ions compared to Cs⁺ and I⁻ ions.
Hydration Energy: The Solvent's Embrace
When an ionic compound is added to water, water molecules, being polar, interact with the ions. The partially positive hydrogen atoms of water molecules are attracted to the negative ions (anions), while the partially negative oxygen atoms are attracted to the positive ions (cations). This interaction is called hydration, and the energy released during the hydration process is called hydration energy. High hydration energy facilitates dissolution. Several factors affect hydration energy:
-
Charge density of the ions: Ions with high charge density (high charge and small size) have stronger interactions with water molecules, resulting in higher hydration energy.
-
Polarity of water: Water's high polarity is crucial for effective hydration of ions. Nonpolar solvents cannot effectively solvate ions.
The Solubility Dance: Lattice Energy vs. Hydration Energy
Solubility is essentially a tug-of-war between lattice energy and hydration energy. If the hydration energy is significantly greater than the lattice energy, the ionic compound will readily dissolve. The ions are effectively "pulled away" from the crystal lattice and surrounded by water molecules. However, if the lattice energy is significantly greater than the hydration energy, the ionic compound will remain largely undissolved. The strong ionic bonds within the crystal lattice prevent the water molecules from effectively separating the ions.
Factors Affecting Solubility of Ionic Compounds
Beyond the basic interplay of lattice and hydration energies, several other factors can influence the solubility of ionic compounds in water:
Temperature: A Thermal Twist
The solubility of most ionic compounds increases with increasing temperature. Higher temperatures provide more kinetic energy to the water molecules, enabling them to overcome the attractive forces within the ionic crystal lattice more effectively. However, there are exceptions to this rule, and some ionic compounds exhibit decreased solubility with increasing temperature.
Pressure: A Subtle Influence
Pressure generally has a minimal effect on the solubility of ionic compounds in water, especially at ambient conditions. The influence of pressure becomes more noticeable at very high pressures.
Common Ion Effect: The Competitive Game
The presence of a common ion in the solution can significantly reduce the solubility of an ionic compound. For example, if you add silver chloride (AgCl) to a solution that already contains a high concentration of chloride ions (Cl⁻), the solubility of AgCl will decrease. This is because the equilibrium between dissolved Ag⁺ and Cl⁻ ions and undissolved AgCl is shifted towards the solid AgCl.
Predicting Solubility: Rules and Guidelines
While predicting the exact solubility of an ionic compound requires precise thermodynamic data, some general guidelines can help determine whether an ionic compound is likely to be soluble or insoluble in water:
- Solubility Rules: A set of general solubility rules is commonly used to predict the solubility of ionic compounds. These rules, however, are not absolute and have exceptions. For instance, most alkali metal salts and nitrates are highly soluble, while most sulfides and phosphates are largely insoluble.
Practical Implications of Ionic Compound Solubility
The solubility of ionic compounds has numerous practical implications:
-
Medicine: The solubility of drugs, many of which are ionic compounds, is critical for their absorption and effectiveness. Poorly soluble drugs may not be efficiently absorbed by the body.
-
Environmental Science: Understanding the solubility of pollutants, often ionic compounds, is essential for assessing their environmental impact and designing effective remediation strategies.
-
Geochemistry: The solubility of minerals plays a crucial role in geological processes, including the formation and weathering of rocks.
-
Industrial Processes: Many industrial processes rely on the solubility of ionic compounds, such as the production of fertilizers, purification of metals, and wastewater treatment.
Beyond Simple Solubility: The Complexities of Aqueous Chemistry
While this discussion has focused on simple solubility, the behavior of ionic compounds in water is often more complex. Factors such as complex ion formation, hydrolysis reactions, and the influence of other dissolved species can significantly affect the observed solubility and chemical properties of the solution. Moreover, solubility is not just about whether a compound dissolves, but also the extent to which it dissolves. Quantitative measures like the solubility product constant (Ksp) provide more precise information about solubility equilibrium.
Conclusion
The question of whether all ionic compounds are soluble in water is definitively answered with a "no." Solubility is a complex phenomenon governed by the interplay between lattice energy and hydration energy, influenced by factors like temperature, pressure, and the presence of common ions. Understanding these factors is crucial across various disciplines and has significant implications in various aspects of science and engineering. While simplified rules provide initial guidance, a more in-depth understanding of the chemical principles and quantitative measures of solubility is necessary for accurate predictions and practical applications. The world of ionic compound solubility is far richer and more nuanced than a simple yes or no answer can convey.
Latest Posts
Latest Posts
-
10 To The Negative 6th Power
Mar 19, 2025
-
What Is The Percentage Of 1 20
Mar 19, 2025
-
What Is The Square Root Of 41
Mar 19, 2025
-
Square Inches In 1 Square Foot
Mar 19, 2025
-
Whats The Square Root Of 26
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Are All Ionic Compounds Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
