All Atoms Of An Element Have The Same Number Of
listenit
Mar 20, 2025 · 6 min read
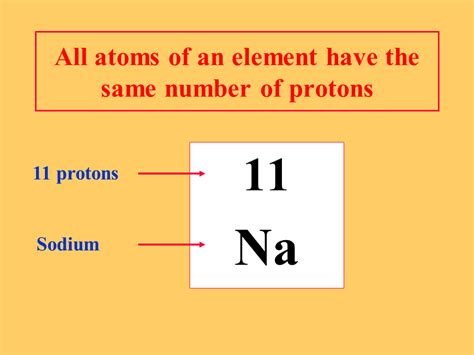
Table of Contents
- All Atoms Of An Element Have The Same Number Of
- Table of Contents
- All Atoms of an Element Have the Same Number of Protons: A Deep Dive into Atomic Structure and Isotopes
- Understanding Atomic Structure: The Trinity of Subatomic Particles
- The Significance of the Atomic Number
- Isotopes: The Same Element, Different Mass
- Understanding Isotopic Abundance
- Applications of Isotopes
- Beyond Protons: Electrons and Chemical Behavior
- Misconceptions about Atomic Structure
- Conclusion: The Unifying Principle of Atomic Number
- Latest Posts
- Latest Posts
- Related Post
All Atoms of an Element Have the Same Number of Protons: A Deep Dive into Atomic Structure and Isotopes
The fundamental building blocks of matter are atoms, and a cornerstone of understanding chemistry and physics lies in grasping the defining characteristic of an element: all atoms of a given element have the same number of protons. This seemingly simple statement underpins our understanding of the periodic table, chemical reactions, and the very nature of matter itself. Let's delve deeper into this crucial concept, exploring its implications and addressing some common misconceptions.
Understanding Atomic Structure: The Trinity of Subatomic Particles
Atoms are not indivisible particles as once thought; they consist of three primary subatomic particles:
- Protons: Positively charged particles residing within the atom's nucleus. The number of protons defines the element.
- Neutrons: Neutrally charged particles also found within the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons generally equals the number of protons in a neutral atom.
The atomic number, denoted by Z, represents the number of protons in an atom's nucleus. This number is unique to each element and is what distinguishes one element from another. For example, hydrogen (H) has an atomic number of 1 (one proton), helium (He) has an atomic number of 2 (two protons), and so on. This is the crux of the statement: all atoms of hydrogen will always have one proton, all atoms of helium will always have two protons, and so forth.
The Significance of the Atomic Number
The atomic number is not merely a label; it dictates an element's chemical properties. The number of protons determines the number of electrons in a neutral atom, and these electrons are the primary participants in chemical bonding. The arrangement of electrons in energy levels (shells) dictates how an atom will interact with other atoms, forming molecules and compounds. This interaction is governed by the principles of quantum mechanics, which describe the behavior of electrons at the atomic level.
The periodic table organizes elements based on their atomic number and recurring chemical properties. Elements with similar electron configurations and therefore similar chemical behaviors are grouped together in columns (groups or families). This arrangement allows us to predict the chemical reactivity and bonding tendencies of elements based on their position in the table.
Isotopes: The Same Element, Different Mass
While all atoms of a given element have the same number of protons, they may differ in the number of neutrons. These variations are called isotopes. Isotopes of the same element have the same atomic number (Z) but different mass numbers (A), where A represents the total number of protons and neutrons in the nucleus.
For example, carbon (C) has an atomic number of 6. The most common isotope is Carbon-12 (¹²C), with 6 protons and 6 neutrons. However, Carbon-13 (¹³C) also exists, with 6 protons and 7 neutrons, and Carbon-14 (¹⁴C), a radioactive isotope, with 6 protons and 8 neutrons. All three are carbon atoms because they all possess 6 protons, but they differ in their mass due to the varying number of neutrons.
Understanding Isotopic Abundance
Isotopes of an element are usually found in nature in specific proportions called isotopic abundance. This abundance varies depending on the element and its origin. For instance, Carbon-12 makes up about 98.9% of naturally occurring carbon, while Carbon-13 comprises about 1.1%. Carbon-14 is present in trace amounts. These abundances are crucial in various applications, including radiocarbon dating.
Applications of Isotopes
The different properties of isotopes lead to a wide range of applications across various scientific fields:
- Radioactive isotopes (like Carbon-14) are used in radioactive dating to determine the age of artifacts and geological formations. The decay rate of these isotopes provides a chronological marker.
- Medical imaging utilizes radioactive isotopes as tracers to visualize internal organs and processes. Techniques like PET (Positron Emission Tomography) scans rely on this principle.
- Nuclear medicine utilizes isotopes for therapeutic purposes, targeting cancerous cells with radiation.
- Industrial applications include using isotopes in gauging thickness, tracing flow patterns, and analyzing material composition.
Beyond Protons: Electrons and Chemical Behavior
While the number of protons defines the element, the number of electrons dictates its chemical behavior. In a neutral atom, the number of electrons equals the number of protons. However, atoms can gain or lose electrons to form ions, which carry a net electrical charge. This process is crucial for chemical bonding.
- Cations: Positively charged ions formed when an atom loses electrons.
- Anions: Negatively charged ions formed when an atom gains electrons.
The formation of ions leads to electrostatic attraction between atoms, resulting in ionic bonds. Alternatively, atoms can share electrons to form covalent bonds, where the shared electrons contribute to the stability of the resulting molecule. The type of bond formed depends on the electronegativity difference between the atoms involved, which is ultimately influenced by their atomic structure and electron configuration – again, rooted in the number of protons.
Misconceptions about Atomic Structure
Several misconceptions often arise concerning atomic structure:
- Atoms are mostly empty space: While the nucleus is tiny compared to the atom's overall size, the space is not entirely empty. Electrons exist in orbitals, regions of high probability of finding an electron, and these orbitals occupy a significant volume.
- The Bohr model is a perfectly accurate representation: The Bohr model, with its orbiting electrons in fixed energy levels, is a simplified model useful for introductory purposes but doesn't fully capture the quantum mechanical nature of electron behavior. The electron's position is probabilistic, not deterministic.
- All isotopes are radioactive: Many isotopes are stable, meaning their nuclei do not spontaneously decay. Only certain isotopes are radioactive, emitting particles or energy as their nuclei transform.
Conclusion: The Unifying Principle of Atomic Number
In conclusion, the fundamental principle that all atoms of an element have the same number of protons is the cornerstone of our understanding of matter. This principle underpins the organization of the periodic table, governs chemical reactions, and explains the diverse properties of elements and their isotopes. While the atomic model has evolved from simplified representations to the complex world of quantum mechanics, the significance of the atomic number remains unchanged – it is the defining characteristic that sets apart one element from all others, making it a truly unifying principle in the world of chemistry and physics. Further exploration into atomic physics, quantum chemistry, and nuclear chemistry builds upon this fundamental concept, revealing the rich complexity and beauty of the atomic world. The more we understand the basic nature of atoms, the better we can harness their properties for technological advancement and scientific discovery.
Latest Posts
Latest Posts
-
What Are The Equivalent Fractions Of 5 8
Mar 22, 2025
-
Can The Pythagorean Theorem Be Used For Any Triangle
Mar 22, 2025
-
What Is 7 25 As A Decimal
Mar 22, 2025
-
35 As A Fraction In Simplest Form
Mar 22, 2025
-
56 1 4 As A Fraction
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about All Atoms Of An Element Have The Same Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
