Alkali Metals With 1 Valence Electron
listenit
Mar 19, 2025 · 6 min read
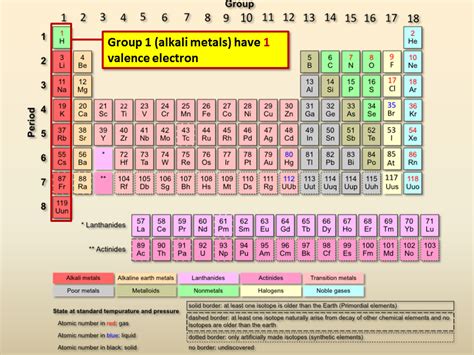
Table of Contents
Alkali Metals: The Lone Wolf of the Periodic Table
Alkali metals, the stars of Group 1 on the periodic table, are renowned for their singular characteristic: a single valence electron. This seemingly simple feature dictates their remarkable reactivity, unique physical properties, and crucial role in various applications, from everyday life to cutting-edge technologies. Let's delve into the fascinating world of these highly reactive elements.
Understanding Valence Electrons: The Key to Reactivity
Before diving into the specifics of alkali metals, let's clarify the significance of valence electrons. These are the electrons located in the outermost shell of an atom. They determine an element's chemical behavior, particularly its ability to form chemical bonds with other atoms. For alkali metals, the presence of just one valence electron is the driving force behind their extraordinary reactivity. This single electron is relatively loosely held, making it readily available to participate in chemical reactions. This ease of electron donation is what defines their chemical character.
The Alkali Metal Family: Lithium, Sodium, Potassium, and Beyond
The alkali metal family consists of six elements: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). While all share the common trait of a single valence electron, their properties vary with increasing atomic number. This variation stems from the increasing number of electron shells, leading to differences in atomic size, ionization energy, and electronegativity.
Lithium (Li): The Lightweight Champion
Lithium, the lightest alkali metal, stands out for its applications in rechargeable batteries, primarily in electric vehicles and portable electronics. Its high energy density makes it a superior choice compared to other metals. Beyond batteries, lithium compounds find use in ceramics, glass manufacturing, and even as a mood stabilizer in medicine.
Sodium (Na): An Everyday Essential
Sodium is undoubtedly the most familiar alkali metal, playing a vital role in our daily lives. Table salt (NaCl), a ubiquitous compound, demonstrates its crucial role in human biology and culinary uses. Sodium also finds applications in various industries, including the production of sodium hydroxide (NaOH), a strong base used in numerous chemical processes, soap manufacturing, and paper production. Sodium lamps, with their characteristic yellow light, are another common example of its use.
Potassium (K): Crucial for Life
Potassium, like sodium, is essential for biological functions. It plays a crucial role in maintaining fluid balance, nerve impulse transmission, and muscle contraction within living organisms. Potassium deficiency can lead to serious health issues, highlighting its importance in our diet. Potassium salts also find use in fertilizers, providing essential nutrients for plant growth.
Rubidium (Rb), Cesium (Cs), and Francium (Fr): The Rarer Members
Rubidium, cesium, and francium are less common and more expensive than their lighter counterparts. Their high reactivity makes them challenging to handle, limiting their widespread applications. However, cesium is used in atomic clocks, taking advantage of its precise atomic transitions, providing highly accurate timekeeping. Francium, being highly radioactive and short-lived, has limited practical applications, primarily serving as a subject of scientific research.
Physical Properties: A Tale of Two Sides
Alkali metals exhibit a unique set of physical properties, directly linked to their electronic structure. They are all soft, silvery-white metals with low melting and boiling points. This softness arises from the weak metallic bonding caused by the single valence electron. The low melting and boiling points reflect the relatively weak attractive forces between the atoms.
Their low density is another noteworthy characteristic, with lithium being the least dense solid metal. This low density is linked to their large atomic radii and relatively weak metallic bonding. Furthermore, alkali metals are excellent conductors of heat and electricity, another consequence of their loosely held valence electrons which can freely move throughout the metal lattice.
Chemical Properties: Highly Reactive Mavericks
The defining characteristic of alkali metals is their high reactivity. This stems from their tendency to readily lose their single valence electron to achieve a stable noble gas configuration. This electron loss leads to the formation of +1 ions, which are extremely stable. Their reactivity increases down the group, reflecting the decreasing ionization energy (the energy required to remove an electron). This means cesium is the most reactive alkali metal.
Reactions with Water: A Dramatic Display
Alkali metals react vigorously with water, producing hydrogen gas and a metal hydroxide. The reaction is particularly dramatic with the heavier alkali metals, often resulting in a flame or even an explosion. This reaction is an excellent demonstration of their high reactivity and the ease with which they lose their valence electron.
The general equation for the reaction is:
2M(s) + 2H₂O(l) → 2MOH(aq) + H₂(g)
Where M represents the alkali metal.
Reactions with Halogens: Forming Ionic Compounds
Alkali metals readily react with halogens (Group 17 elements), forming ionic compounds known as alkali halides. These reactions involve the transfer of the alkali metal's valence electron to the halogen atom, forming a stable ionic bond. Sodium chloride (NaCl), the common table salt, is a prime example of such an ionic compound.
Reactions with Oxygen: Forming Oxides and Peroxides
The reaction of alkali metals with oxygen is complex and depends on the specific metal and reaction conditions. Generally, they form oxides or peroxides. Lithium forms lithium oxide (Li₂O), while sodium forms sodium peroxide (Na₂O₂). The heavier alkali metals can form superoxides (e.g., KO₂).
Applications: From Batteries to Biology
The unique properties of alkali metals have led to a wide range of applications in various fields. Let’s explore some key examples:
- Batteries: Lithium-ion batteries dominate the portable electronics and electric vehicle markets due to lithium's high energy density.
- Lighting: Sodium lamps are commonly used in street lighting due to their high efficiency and characteristic yellow light.
- Medicine: Lithium compounds find use as mood stabilizers in the treatment of bipolar disorder. Potassium is crucial for maintaining electrolyte balance in the human body.
- Industry: Sodium hydroxide is a key chemical in various industrial processes, including soap manufacturing, paper production, and the refining of metals.
- Agriculture: Potassium salts are essential components of fertilizers, providing potassium, a crucial nutrient for plant growth.
- Atomic Clocks: Cesium atomic clocks leverage the precise atomic transitions of cesium to provide extremely accurate timekeeping.
Environmental Considerations: Handling Reactivity with Care
The high reactivity of alkali metals presents both opportunities and challenges. Their use in various applications requires careful handling and storage to prevent accidents. Their reactions with water and air necessitate safety precautions to avoid fire or explosion hazards. Furthermore, the environmental impact of alkali metal mining and processing needs to be carefully considered and mitigated to ensure sustainable practices.
Conclusion: The Enduring Significance of Alkali Metals
Alkali metals, despite their seemingly simple structure, play a significant role in our world. Their unique properties, driven by their single valence electron, have found applications across various fields, from everyday life to cutting-edge technologies. Understanding their reactivity and characteristics is crucial for both safe handling and harnessing their potential benefits in diverse applications. Further research into their properties and potential applications will continue to shape technological advancements and scientific discoveries in the future. Their ongoing study is a testament to the enduring fascination and importance of these unique elements.
Latest Posts
Latest Posts
-
How Many Ounces Are In 1 05 Qt
May 09, 2025
-
How To Determine Most Stable Chair Conformation
May 09, 2025
-
A Pair Of Pants With A Marked Price Of 35 00
May 09, 2025
-
18 Ones 9 Tens 2 Hundreds
May 09, 2025
-
Potassium Hydrogen Phthalate And Sodium Hydroxide
May 09, 2025
Related Post
Thank you for visiting our website which covers about Alkali Metals With 1 Valence Electron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.